Page 80 - Fundamentals of Gas Shale Reservoirs
P. 80
60 GEOCHEMICAL ASSESSMENT OF UNCONVENTIONAL SHALE GAS RESOURCE SYSTEMS
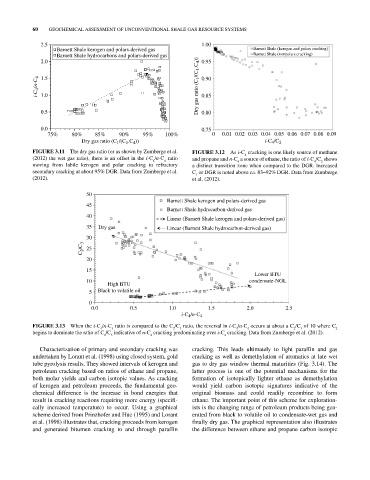
2.5 1.00
Barnett Shale kerogen and polars-derived gas Barnett Shale (kerogen and polars cracking)
Barnett Shale hydrocarbons and polars-derived gas Barnett Shale (nonpolars cracking)
2.0 0.95
i-C 4 /n-C 4 1.5 Dry gas ratio (C 1 /(C 1 .C 4 )) 0.90
1.0
0.85
0.5 0.80
0.0 0.75
75% 80% 85% 90% 95% 100% 0 0.01 0.02 0.03 0.04 0.05 0.06 0.07 0.08 0.09
Dry gas ratio (C /(C .C )) i-C 4 /C 2
1
4
1
FIGURE 3.11 The dry gas ratio (or as shown by Zumberge et al. FIGURE 3.12 As i‐C cracking is one likely source of methane
4
(2012) the wet gas ratio), there is an offset in the i‐C /n‐C ratio and propane and n‐C a source of ethane, the ratio of i‐C /C shows
4
4
4
4
2
moving from labile kerogen and polar cracking to refractory a distinct transition zone when compared to the DGR. Increased
secondary cracking at about 95% DGR. Data from Zumberge et al. C or DGR is noted above ca. 83–92% DGR. Data from Zumberge
1
(2012). et al. (2012).
50
Barnett Shale kerogen and polars-derived gas
45
Barnett Shale hydrocarbon-derived gas
40
Linear (Barnett Shale kerogen and polars-derived gas)
35 Dry gas Linear (Barnett Shale hydrocarbon-derived gas)
30
C 2 /C 3 25
20
15
Lower BTU
10 condensate-NGL
High BTU
5 Black to volatile oil
0
0.0 0.5 1.0 1.5 2.0 2.5
i-C 4 /n-C 4
FIGURE 3.13 When the i‐C /n‐C ratio is compared to the C /C ratio, the reversal in i‐C /n‐C occurs at about a C /C of 10 where C
3
2
4
4
4
2
3
2
4
begins to dominate the ratio of C /C indicative of n‐C cracking predominating over i‐C cracking. Data from Zumberge et al. (2012).
2 3 4 4
Characterization of primary and secondary cracking was cracking. This leads ultimately to light paraffin and gas
undertaken by Lorant et al. (1998) using closed system, gold cracking as well as demethylation of aromatics at late wet
tube pyrolysis results. They showed intervals of kerogen and gas to dry gas window thermal maturities (Fig. 3.14). The
petroleum cracking based on ratios of ethane and propane, latter process is one of the potential mechanisms for the
both molar yields and carbon isotopic values. As cracking formation of isotopically lighter ethane as demethylation
of kerogen and petroleum proceeds, the fundamental geo would yield carbon isotopic signatures indicative of the
chemical difference is the increase in bond energies that original biomass and could readily recombine to form
result in cracking reactions requiring more energy (specifi ethane. The important point of this scheme for exploration
cally increased temperature) to occur. Using a graphical ists is the changing range of petroleum products being gen
scheme derived from Prinzhofer and Huc (1995) and Lorant erated from black to volatile oil to condensate‐wet gas and
et al. (1998) illustrates that, cracking proceeds from kerogen finally dry gas. The graphical representation also illustrates
and generated bitumen cracking to and through paraffin the difference between ethane and propane carbon isotopic