Page 218 - Hydrogeology Principles and Practice
P. 218
HYDC06 12/5/05 5:33 PM Page 201
Groundwater quality and contaminant hydrogeology 201
ion; hence the factors 2.5 and 4.1 are included in the
hardness relation (Freeze & Cherry 1979).
Where reported, carbonate hardness includes that
−
part of the total hardness equivalent to the HCO and
3
2−
CO content (or alkalinity). If the total hardness
3
exceeds the alkalinity, the excess is termed the non-
carbonate hardness and is a measure of the calcium
and magnesium sulphates. In older publications, the
terms ‘temporary’ and ‘permanent’ are used in place
of ‘carbonate’ and ‘non-carbonate’. Temporary hard-
ness reflects the fact that the ions responsible may
be precipitated by boiling, such that:
−
2+
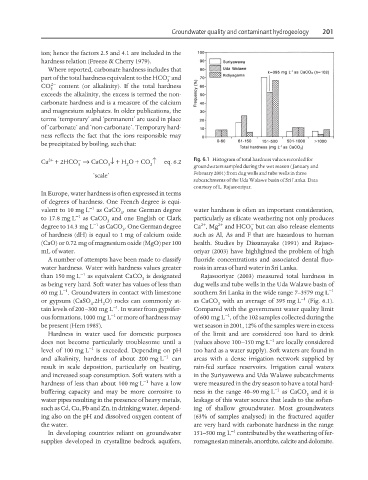
Ca + 2HCO → CaCO ↓+ H O + CO ↑ eq. 6.2 Fig. 6.1 Histogram of total hardness values recorded for
3 3 2 2
groundwaters sampled during the wet season ( January and
‘scale’ February 2001) from dug wells and tube wells in three
subcatchments of the Uda Walawe basin of Sri Lanka. Data
courtesy of L. Rajasooriyar.
In Europe, water hardness is often expressed in terms
of degrees of hardness. One French degree is equi-
valent to 10 mg L −1 as CaCO , one German degree water hardness is often an important consideration,
3
to 17.8 mg L −1 as CaCO and one English or Clark particularly as silicate weathering not only produces
3
−
2+
2+
−1
degree to 14.3 mg L as CaCO . One German degree Ca , Mg and HCO but can also release elements
3 3
of hardness (dH) is equal to 1 mg of calcium oxide such as Al, As and F that are hazardous to human
(CaO) or 0.72 mg of magnesium oxide (MgO) per 100 health. Studies by Dissanayake (1991) and Rajaso-
mL of water. oriyar (2003) have highlighted the problem of high
A number of attempts have been made to classify fluoride concentrations and associated dental fluo-
water hardness. Water with hardness values greater rosis in areas of hard water in Sri Lanka.
than 150 mg L −1 as equivalent CaCO is designated Rajasooriyar (2003) measured total hardness in
3
as being very hard. Soft water has values of less than dug wells and tube wells in the Uda Walawe basin of
−1
60 mg L . Groundwaters in contact with limestone southern Sri Lanka in the wide range 7–3579 mg L −1
or gypsum (CaSO .2H O) rocks can commonly at- as CaCO with an average of 395 mg L −1 (Fig. 6.1).
4 2 3
−1
tain levels of 200–300 mg L . In water from gypsifer- Compared with the government water quality limit
−1
−1
ous formations, 1000 mg L or more of hardness may of 600 mg L , of the 102 samples collected during the
be present (Hem 1985). wet season in 2001, 12% of the samples were in excess
Hardness in water used for domestic purposes of the limit and are considered too hard to drink
−1
does not become particularly troublesome until a (values above 100–150 mg L are locally considered
level of 100 mg L −1 is exceeded. Depending on pH too hard as a water supply). Soft waters are found in
and alkalinity, hardness of about 200 mg L −1 can areas with a dense irrigation network supplied by
result in scale deposition, particularly on heating, rain-fed surface reservoirs. Irrigation canal waters
and increased soap consumption. Soft waters with a in the Suriyawewa and Uda Walawe subcatchments
hardness of less than about 100 mg L −1 have a low were measured in the dry season to have a total hard-
buffering capacity and may be more corrosive to ness in the range 40–90 mg L −1 as CaCO and it is
3
water pipes resulting in the presence of heavy metals, leakage of this water source that leads to the soften-
such as Cd, Cu, Pb and Zn, in drinking water, depend- ing of shallow groundwater. Most groundwaters
ing also on the pH and dissolved oxygen content of (63% of samples analysed) in the fractured aquifer
the water. are very hard with carbonate hardness in the range
−1
In developing countries reliant on groundwater 151–500 mg L contributed by the weathering of fer-
supplies developed in crystalline bedrock aquifers, romagnesian minerals, anorthite, calcite and dolomite.