Page 219 - Hydrogeology Principles and Practice
P. 219
HYDC06 12/5/05 5:33 PM Page 202
202 Chapter Six
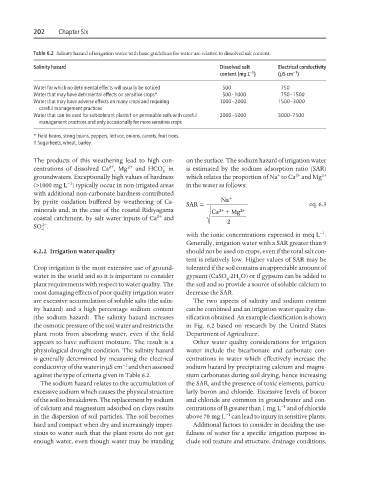
Table 6.2 Salinity hazard of irrigation water with basic guidelines for water use relative to dissolved salt content.
Salinity hazard Dissolved salt Electrical conductivity
−1
−1
content (mg L ) (mScm )
Water for which no detrimental effects will usually be noticed 500 750
Water that may have detrimental effects on sensitive crops* 500–1000 750–1500
Water that may have adverse effects on many crops and requiring 1000–2000 1500–3000
careful management practices
Water that can be used for salt-tolerant plants† on permeable soils with careful 2000–5000 3000–7500
management practices and only occasionally for more sensitive crops
* Field beans, string beans, peppers, lettuce, onions, carrots, fruit trees.
† Sugarbeets, wheat, barley.
The products of this weathering lead to high con- on the surface. The sodium hazard of irrigation water
2+ 2+ −
centrations of dissolved Ca , Mg and HCO in is estimated by the sodium adsorption ratio (SAR)
3
2+
+
groundwaters. Exceptionally high values of hardness which relates the proportion of Na to Ca and Mg 2+
−1
(>1000 mg L ) typically occur in non-irrigated areas in the water as follows:
with additional non-carbonate hardness contributed +
by pyrite oxidation buffered by weathering of Ca- = Na
SAR eq. 6.3
minerals and, in the case of the coastal Ridiyagama Ca + Mg + 2
+ 2
2+
coastal catchment, by salt water inputs of Ca and
2− 2
SO .
4
−1
with the ionic concentrations expressed in meq L .
Generally, irrigation water with a SAR greater than 9
6.2.2 Irrigation water quality should not be used on crops, even if the total salt con-
tent is relatively low. Higher values of SAR may be
Crop irrigation is the most extensive use of ground- tolerated if the soil contains an appreciable amount of
water in the world and so it is important to consider gypsum (CaSO ⋅2H O) or if gypsum can be added to
4 2
plant requirements with respect to water quality. The the soil and so provide a source of soluble calcium to
most damaging effects of poor quality irrigation water decrease the SAR.
are excessive accumulation of soluble salts (the salin- The two aspects of salinity and sodium content
ity hazard) and a high percentage sodium content can be combined and an irrigation water quality clas-
(the sodium hazard). The salinity hazard increases sification obtained. An example classification is shown
the osmotic pressure of the soil water and restricts the in Fig. 6.2 based on research by the United States
plant roots from absorbing water, even if the field Department of Agriculture.
appears to have sufficient moisture. The result is a Other water quality considerations for irrigation
physiological drought condition. The salinity hazard water include the bicarbonate and carbonate con-
is generally determined by measuring the electrical centrations in water which effectively increase the
−1
conductivity of the water in µScm and then assessed sodium hazard by precipitating calcium and magne-
against the type of criteria given in Table 6.2. sium carbonates during soil drying, hence increasing
The sodium hazard relates to the accumulation of the SAR, and the presence of toxic elements, particu-
excessive sodium which causes the physical structure larly boron and chloride. Excessive levels of boron
of the soil to breakdown. The replacement by sodium and chloride are common in groundwater and con-
−1
of calcium and magnesium adsorbed on clays results centrations of B greater than 1 mg L and of chloride
−1
in the dispersion of soil particles. The soil becomes above 70 mg L can lead to injury in sensitive plants.
hard and compact when dry and increasingly imper- Additional factors to consider in deciding the use-
vious to water such that the plant roots do not get fulness of water for a specific irrigation purpose in-
enough water, even though water may be standing clude soil texture and structure, drainage conditions,