Page 221 - Hydrogeology Principles and Practice
P. 221
HYDC06 12/5/05 5:33 PM Page 204
204 Chapter Six
2
∂C ∂ C
=− D* eq. 6.4
∂t ∂x 2
where D*is the molecular diffusion coefficient for the
solute in a porous material. The analytical solution
for an instantaneous step-change in solute concentra-
tion, C, for an infinite aquifer space is given by:
C ⎛ x ⎞
=
erfc ⎜ ⎟ eq. 6.5
C ⎝ 2 Dt ⎠
*
0
where erfc is the complementary error function (see
Appendix 8 for tabulated values of erf (error function)
and erfc), C is the initial concentration at x = 0 at
0
time t = 0, and C is the concentration measured at
position x at time t. A graphical solution to equation
6.5 is shown in Fig. 6.5 for values of diffusion co-
2 −1
efficient equal to 10 −10 and 10 −11 m s . Even after
10,000 years, the diffusive breakthrough of contamin-
ant with a relative concentration of 0.01 (or 1% of the
initial concentration) has only reached about 25 m
from the pollution source.
Of greater importance in terms of contaminant
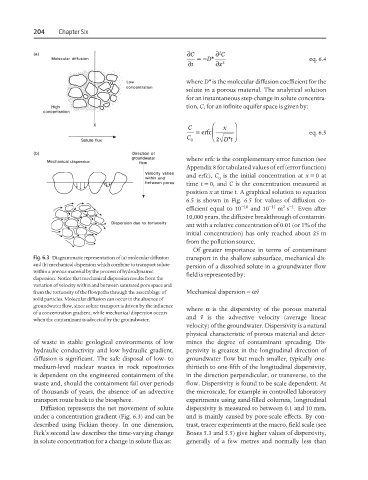
Fig. 6.3 Diagrammatic representation of (a) molecular diffusion transport in the shallow subsurface, mechanical dis-
and (b) mechanical dispersion which combine to transport solute persion of a dissolved solute in a groundwater flow
within a porous material by the process of hydrodynamic field is represented by:
dispersion. Notice that mechanical dispersion results from the
variation of velocity within and between saturated pore space and
from the tortuosity of the flowpaths through the assemblage of Mechanical dispersion = αV
solid particles. Molecular diffusion can occur in the absence of
groundwater flow, since solute transport is driven by the influence where α is the dispersivity of the porous material
of a concentration gradient, while mechanical dispersion occurs
when the contaminant is advected by the groundwater. and V is the advective velocity (average linear
velocity) of the groundwater. Dispersivity is a natural
physical characteristic of porous material and deter-
of waste in stable geological environments of low mines the degree of contaminant spreading. Dis-
hydraulic conductivity and low hydraulic gradient, persivity is greatest in the longitudinal direction of
diffusion is significant. The safe disposal of low- to groundwater flow but much smaller, typically one-
medium-level nuclear wastes in rock repositories thirtieth to one-fifth of the longitudinal dispersivity,
is dependent on the engineered containment of the in the direction perpendicular, or transverse, to the
waste and, should the containment fail over periods flow. Dispersivity is found to be scale dependent. At
of thousands of years, the absence of an advective the microscale, for example in controlled laboratory
transport route back to the biosphere. experiments using sand-filled columns, longitudinal
Diffusion represents the net movement of solute dispersivity is measured to between 0.1 and 10 mm,
under a concentration gradient (Fig. 6.3) and can be and is mainly caused by pore-scale effects. By con-
described using Fickian theory. In one dimension, trast, tracer experiments at the macro, field scale (see
Fick’s second law describes the time-varying change Boxes 5.3 and 5.5) give higher values of dispersivity,
in solute concentration for a change in solute flux as: generally of a few metres and normally less than