Page 148 - Sami Franssila Introduction to Microfabrication
P. 148
Etching 127
◦
Table 11.6 Etch product boiling points (T bp , C)
SiF 4 −90 SiCl 4 −70 CO 2 −56
−206 190 −133
NF 3 AlCl 3 PH 3
WF 6 2.5 GaCl 3 78 AsH 3 −116
110 −25
WOF 4 TiCl 4
TaF 5 96.8 WOCl 4 211 SiBr 2 5.4
17.5 275
MoF 6 WCl 6
MoOF 4 98 InCl 2 235
NbF 5 72 MoCl 5 194
PtCl 4 370d (a)
PbCl4 −15
Cr(CO) 6 110d
Note: d – decomposition
Table 11.7 Non-etchable reaction products
◦
(T bp , C)
CuCl 2 620 TiF 4 >400
950d 855
CuF 2 PbF 2
CrCl 2 824 CrF 2 1100
AlF 3 1290s TiF 3 1200 (b)
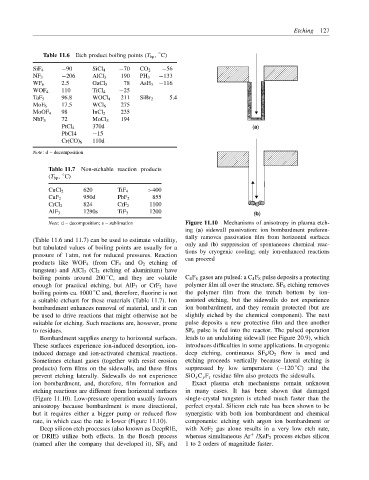
Note: d – decomposition; s – sublimation Figure 11.10 Mechanisms of anisotropy in plasma etch-
ing (a) sidewall passivation: ion bombardment preferen-
tially removes passivation film from horizontal surfaces
(Table 11.6 and 11.7) can be used to estimate volatility,
but tabulated values of boiling points are usually for a only and (b) suppression of spontaneous chemical reac-
pressure of 1 atm, not for reduced pressures. Reaction tions by cryogenic cooling; only ion-enhanced reactions
can proceed
products like WOF 4 (from CF 4 and O 2 etching of
tungsten) and AlCl 3 (Cl 2 etching of aluminium) have
◦
boiling points around 200 C, and they are volatile C 4 F 8 gases are pulsed: a C 4 F 8 pulse deposits a protecting
enough for practical etching, but AlF 3 or CrF 2 have polymer film all over the structure. SF 6 etching removes
◦
boiling points ca. 1000 C and, therefore, fluorine is not the polymer film from the trench bottom by ion-
a suitable etchant for these materials (Table 11.7). Ion assisted etching, but the sidewalls do not experience
bombardment enhances removal of material, and it can ion bombardment, and they remain protected (but are
be used to drive reactions that might otherwise not be slightly etched by the chemical component). The next
suitable for etching. Such reactions are, however, prone pulse deposits a new protective film and then another
to residues. SF 6 pulse is fed into the reactor. The pulsed operation
Bombardment supplies energy to horizontal surfaces. leads to an undulating sidewall (see Figure 20.9), which
These surfaces experience ion-induced desorption, ion- introduces difficulties in some applications. In cryogenic
induced damage and ion-activated chemical reactions. deep etching, continuous SF 6 /O 2 flow is used and
Sometimes etchant gases (together with resist erosion etching proceeds vertically because lateral etching is
◦
products) form films on the sidewalls, and these films suppressed by low temperature (−120 C) and the
prevent etching laterally. Sidewalls do not experience SiO x C y F z residue film also protects the sidewalls.
ion bombardment, and, therefore, film formation and Exact plasma etch mechanisms remain unknown
etching reactions are different from horizontal surfaces in many cases. It has been shown that damaged
(Figure 11.10). Low-pressure operation usually favours single-crystal tungsten is etched much faster than the
anisotropy because bombardment is more directional, perfect crystal. Silicon etch rate has been shown to be
but it requires either a bigger pump or reduced flow synergistic with both ion bombardment and chemical
rate, in which case the rate is lower (Figure 11.10). components: etching with argon ion bombardment or
Deep silicon etch processes (also known as DeepRIE, with XeF 2 gas alone results in a very low etch rate,
+
or DRIE) utilize both effects. In the Bosch process whereas simultaneous Ar /XeF 2 process etches silicon
(named after the company that developed it), SF 6 and 1 to 2 orders of magnitude faster.