Page 189 - Sami Franssila Introduction to Microfabrication
P. 189
168 Introduction to Microfabrication
1000 16.3 CHEMISTRY OF CMP
Cu polish rate (nm/min) 600 ponents: in addition to the mechanical pressure, chemi-
In chemical–mechanical polishing, there are two com-
800
cal modifications and etching take place. For instance, a
tungsten surface is turned into tungsten oxide according
400
to the following equation:
200
0 W + 6Fe(CN) 6 3− + 3H 2 O −→ 4− +
0 5 10 15 20 25 WO 3 + 6Fe(CN) 6 + 6H
Velocity (cm/sec)
Tungsten oxide has two important roles: it is a protective
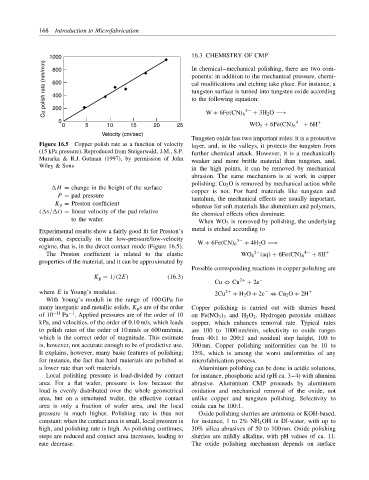
Figure 16.5 Copper polish rate as a function of velocity layer, and, in the valleys, it protects the tungsten from
(15 kPa pressure). Reproduced from Steigerwald, J.M., S.P. further chemical attack. However, it is a mechanically
Murarka & R.J. Gutman (1997), by permission of John weaker and more brittle material than tungsten, and,
Wiley & Sons
in the high points, it can be removed by mechanical
abrasion. The same mechanism is at work in copper
polishing: Cu 2 O is removed by mechanical action while
H = change in the height of the surface
copper is not. For hard materials like tungsten and
P = pad pressure tantalum, the mechanical effects are usually important,
K p = Preston coefficient
whereas for soft materials like aluminium and polymers,
( s/ t) = linear velocity of the pad relative
the chemical effects often dominate.
to the wafer. When WO 3 is removed by polishing, the underlying
metal is etched according to
Experimental results show a fairly good fit for Preston’s
equation, especially in the low-pressure/low-velocity
3−
W + 6Fe(CN) 6 + 4H 2 O −→
regime, that is, in the direct contact mode (Figure 16.5).
The Preston coefficient is related to the elastic WO 4 2− (aq) + 6Fe(CN) 6 4− + 8H +
properties of the material, and it can be approximated by
Possible corresponding reactions in copper polishing are
K p = 1/(2E) (16.3)
Cu ⇔ Cu 2+ + 2e −
where E is Young’s modulus. 2Cu 2+ + H 2 O + 2e ⇔ Cu 2 O + 2H +
−
With Young’s moduli in the range of 100 GPa for
many inorganic and metallic solids, K p s are of the order Copper polishing is carried out with slurries based
−1
of 10 −11 Pa . Applied pressures are of the order of 10 on Fe(NO 3 ) 3 and H 2 O 2 . Hydrogen peroxide oxidizes
kPa, and velocities, of the order of 0.10 m/s, which leads copper, which enhances removal rate. Typical rates
to polish rates of the order of 10 nm/s or 600 nm/min, are 100 to 1000 nm/min, selectivity to oxide ranges
which is the correct order of magnitude. This estimate from 40:1 to 200:1 and residual step height, 100 to
is, however, not accurate enough to be of predictive use. 300 nm. Copper polishing uniformities can be 10 to
It explains, however, many basic features of polishing; 15%, which is among the worst uniformities of any
for instance, the fact that hard materials are polished at microfabrication process.
a lower rate than soft materials. Aluminium polishing can be done in acidic solutions,
Local polishing pressure is load-divided by contact for instance, phosphoric acid (pH ca. 3–4) with alumina
area. For a flat wafer, pressure is low because the abrasive. Aluminium CMP proceeds by aluminium
load is evenly distributed over the whole geometrical oxidation and mechanical removal of the oxide, not
area, but on a structured wafer, the effective contact unlike copper and tungsten polishing. Selectivity to
area is only a fraction of wafer area, and the local oxide can be 100:1.
pressure is much higher. Polishing rate is thus not Oxide polishing slurries are ammonia or KOH-based,
constant: when the contact area is small, local pressure is for instance, 1 to 2% NH 4 OH in DI-water, with up to
high, and polishing rate is high. As polishing continues, 30% silica abrasives of 50 to 100 nm. Oxide polishing
steps are reduced and contact area increases, leading to slurries are mildly alkaline, with pH values of ca. 11.
rate decrease. The oxide polishing mechanism depends on surface