Page 217 - Sami Franssila Introduction to Microfabrication
P. 217
196 Introduction to Microfabrication
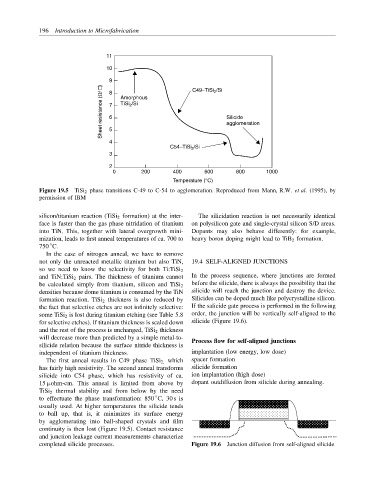
11
10
9 8 C49−TiSi 2 /Si
Sheet resistance (Ω/ ) 7 6 Amorphous Silicide
TiSi 2 /Si
agglomeration
4 5
C54−TiSi 2 /Si
3
2
0 200 400 600 800 1000
Temperature (°C)
Figure 19.5 TiSi 2 phase transitions C-49 to C-54 to agglomeration. Reproduced from Mann, R.W. et al. (1995), by
permission of IBM
silicon/titanium reaction (TiSi 2 formation) at the inter- The silicidation reaction is not necessarily identical
face is faster than the gas phase nitridation of titanium on polysilicon gate and single-crystal silicon S/D areas.
into TiN. This, together with lateral overgrowth mini- Dopants may also behave differently: for example,
mization, leads to first anneal temperatures of ca. 700 to heavy boron doping might lead to TiB 2 formation.
◦
750 C.
In the case of nitrogen anneal, we have to remove
not only the unreacted metallic titanium but also TiN, 19.4 SELF-ALIGNED JUNCTIONS
so we need to know the selectivity for both Ti:TiSi 2
and TiN:TiSi 2 pairs. The thickness of titanium cannot In the process sequence, where junctions are formed
before the silicide, there is always the possibility that the
be calculated simply from titanium, silicon and TiSi 2
densities because dome titanium is consumed by the TiN silicide will reach the junction and destroy the device.
formation reaction. TiSi 2 thickness is also reduced by Silicides can be doped much like polycrystalline silicon.
the fact that selective etches are not infinitely selective: If the salicide gate process is performed in the following
some TiSi 2 is lost during titanium etching (see Table 5.8 order, the junction will be vertically self-aligned to the
for selective etches). If titanium thickness is scaled down silicide (Figure 19.6).
and the rest of the process is unchanged, TiSi 2 thickness
will decrease more than predicted by a simple metal-to- Process flow for self-aligned junctions
silicide relation because the surface nitride thickness is
independent of titanium thickness. implantation (low energy, low dose)
The first anneal results in C49 phase TiSi 2, which spacer formation
has fairly high resistivity. The second anneal transforms silicide formation
silicide into C54 phase, which has resistivity of ca. ion implantation (high dose)
15 µohm-cm. This anneal is limited from above by dopant outdiffusion from silicide during annealing.
TiSi 2 thermal stability and from below by the need
◦
to effectuate the phase transformation: 850 C, 30 s is
usually used. At higher temperatures the silicide tends
to ball up, that is, it minimizes its surface energy
by agglomerating into ball-shaped crystals and film
continuity is then lost (Figure 19.5). Contact resistance
and junction leakage current measurements characterize
completed silicide processes. Figure 19.6 Junction diffusion from self-aligned silicide