Page 353 - Sami Franssila Introduction to Microfabrication
P. 353
332 Introduction to Microfabrication
Process 3
Deposition rate 1 4
2
window
Temperature
Figure 33.3 Process window for ALD (see text for
details)
ratio contact hole, or scaled down gate oxides. In both
cases, a few nanometres are enough.
ALD operating temperature is limited from below
by two mechanisms (numbers refer to Figure 33.3):
low temperature leads to a low reaction rate (1), and
precursor condensation on the surface leads to excessive
deposition (2). The former leads to less than the
monolayer deposition, and the latter to non-self-limiting
deposition of unwanted composition. Upper operating
temperature is also limited by two mechanisms: thermal
decomposition of the precursors, which results in
deposition in the normal CVD fashion (3), and high
re-evaporation rate, which leads to sub-monolayer
growth per cycle (4). Under the right conditions, a
uniform monolayer (or sub-monolayer) formation is
observed.
ALD is a variant of CVD, but its deposition mecha-
nism is definitely different: in CVD, the deposition rate
is strongly temperature dependent, but in ALD there is
a (wide) process window in which the rate is indepen-
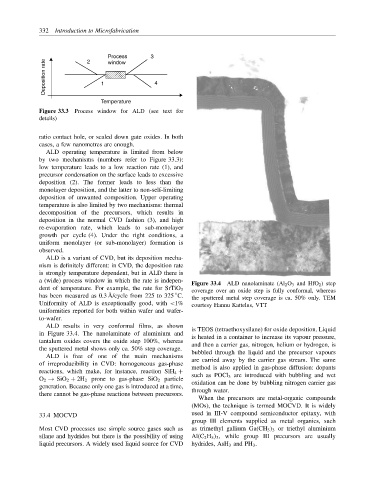
Figure 33.4 ALD nanolaminate (Al 2 O 3 and HfO 2 ) step
dent of temperature. For example, the rate for SrTiO 3 coverage over an oxide step is fully conformal, whereas
has been measured as 0.3 ˚ A/cycle from 225 to 325 C. the sputtered metal step coverage is ca. 50% only. TEM
◦
Uniformity of ALD is exceptionally good, with <1% courtesy Hannu Kattelus, VTT
uniformities reported for both within wafer and wafer-
to-wafer.
ALD results in very conformal films, as shown is TEOS (tetraethoxysilane) for oxide deposition. Liquid
in Figure 33.4. The nanolaminate of aluminium and is heated in a container to increase its vapour pressure,
tantalum oxides covers the oxide step 100%, whereas and then a carrier gas, nitrogen, helium or hydrogen, is
the sputtered metal shows only ca. 50% step coverage. bubbled through the liquid and the precursor vapours
ALD is free of one of the main mechanisms
of irreproducibility in CVD: homogeneous gas-phase are carried away by the carrier gas stream. The same
method is also applied in gas-phase diffusion: dopants
reactions, which make, for instance, reaction SiH 4 +
such as POCl 3 are introduced with bubbling and wet
O 2 → SiO 2 + 2H 2 prone to gas-phase SiO 2 particle
oxidation can be done by bubbling nitrogen carrier gas
generation. Because only one gas is introduced at a time,
through water.
there cannot be gas-phase reactions between precursors.
When the precursors are metal-organic compounds
(MOs), the technique is termed MOCVD. It is widely
33.4 MOCVD used in III-V compound semiconductor epitaxy, with
group III elements supplied as metal organics, such
Most CVD processes use simple source gases such as as trimethyl gallium Ga(CH 3 ) 3 or triethyl aluminium
silane and hydrides but there is the possibility of using Al(C 2 H 5 ) 3 , while group III precursors are usually
liquid precursors. A widely used liquid source for CVD hydrides, AsH 3 and PH 3 .