Page 57 - Sami Franssila Introduction to Microfabrication
P. 57
36 Introduction to Microfabrication
100000
10 000 p-type
1000 n-type
Resistivity (ohm-cm) 10
100
1
0.1
0.01
0.001
0.0001
1.E+12 1.E+13 1.E+14 1.E+15 1.E+16 1.E+17 1.E+18 1.E+19 1.E+20 1.E+21
−3
Dopant concentration (cm )
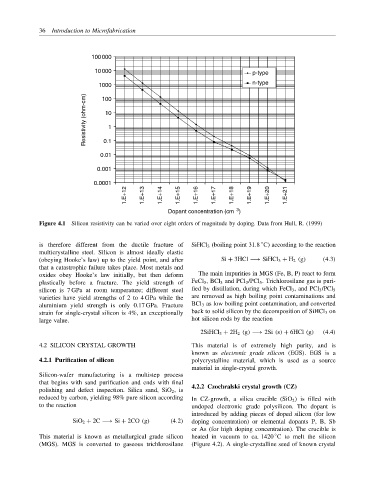
Figure 4.1 Silicon resistivity can be varied over eight orders of magnitude by doping. Data from Hull, R. (1999)
◦
is therefore different from the ductile fracture of SiHCl 3 (boiling point 31.8 C) according to the reaction
multicrystalline steel. Silicon is almost ideally elastic
(obeying Hooke’s law) up to the yield point, and after Si + 3HCl −→ SiHCl 3 + H 2 (g) (4.3)
that a catastrophic failure takes place. Most metals and
oxides obey Hooke’s law initially, but then deform The main impurities in MGS (Fe, B, P) react to form
plastically before a fracture. The yield strength of FeCl 3 , BCl 3 and PCl 3 /PCl 5 . Trichlorosilane gas is puri-
silicon is 7 GPa at room temperature; different steel fied by distillation, during which FeCl 3 , and PCl 3 /PCl 5
varieties have yield strengths of 2 to 4 GPa while the are removed as high boiling point contaminations and
aluminium yield strength is only 0.17 GPa. Fracture BCl 3 as low boiling point contamination, and converted
strain for single-crystal silicon is 4%, an exceptionally back to solid silicon by the decomposition of SiHCl 3 on
large value. hot silicon rods by the reaction
2SiHCl 3 + 2H 2 (g) −→ 2Si (s) + 6HCl (g) (4.4)
4.2 SILICON CRYSTAL GROWTH This material is of extremely high purity, and is
known as electronic grade silicon (EGS). EGS is a
4.2.1 Purification of silicon polycrystalline material, which is used as a source
material in single-crystal growth.
Silicon-wafer manufacturing is a multistep process
that begins with sand purification and ends with final
polishing and defect inspection. Silica sand, SiO 2 , is 4.2.2 Czochralski crystal growth (CZ)
reduced by carbon, yielding 98% pure silicon according In CZ-growth, a silica crucible (SiO 2 ) is filled with
to the reaction undoped electronic grade polysilicon. The dopant is
introduced by adding pieces of doped silicon (for low
SiO 2 + 2C −→ Si + 2CO (g) (4.2) doping concentration) or elemental dopants P, B, Sb
or As (for high doping concentration). The crucible is
This material is known as metallurgical grade silicon heated in vacuum to ca. 1420 C to melt the silicon
◦
(MGS). MGS is converted to gaseous trichlorosilane (Figure 4.2). A single-crystalline seed of known crystal