Page 322 - Lindens Handbook of Batteries
P. 322
13.28 PrImArY BATTErIES
1.3
1.2
Voltage, V 1.1
1.0
0.9
15
12 (I L )
Current, mA 9 6 (I ave )
3 a) 15 mA-1s/ b) 15 mA-1s/ c) 15 mA-1 s/ d) 15 mA-1s/
5 mA-9 s 5 mA-4 s 5 mA-2 s 5 mA-1 s
0
0 30 60 90 120
Time, s
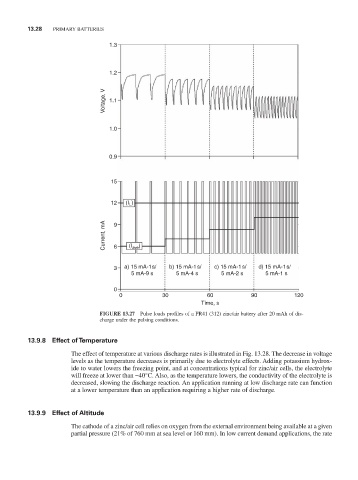
FiGURE 13.27 Pulse loads profiles of a Pr41 (312) zinc/air battery after 20 mAh of dis-
charge under the pulsing conditions.
13.9.8 effect of temperature
The effect of temperature at various discharge rates is illustrated in Fig. 13.28. The decrease in voltage
levels as the temperature decreases is primarily due to electrolyte effects. Adding potassium hydrox-
ide to water lowers the freezing point, and at concentrations typical for zinc/air cells, the electrolyte
will freeze at lower than -40°C. Also, as the temperature lowers, the conductivity of the electrolyte is
decreased, slowing the discharge reaction. An application running at low discharge rate can function
at a lower temperature than an application requiring a higher rate of discharge.
13.9.9 effect of altitude
The cathode of a zinc/air cell relies on oxygen from the external environment being available at a given
partial pressure (21% of 760 mm at sea level or 160 mm). In low current demand applications, the rate