Page 324 - Lindens Handbook of Batteries
P. 324
13.30 PrImArY BATTErIES
18
Limiting current (0.9 V)
16
Current, mA 14
12
Current density (1.1 V)
10
8
0 5,000 10,000 15,000 20,000 25,000 30,000
Altitude, ft
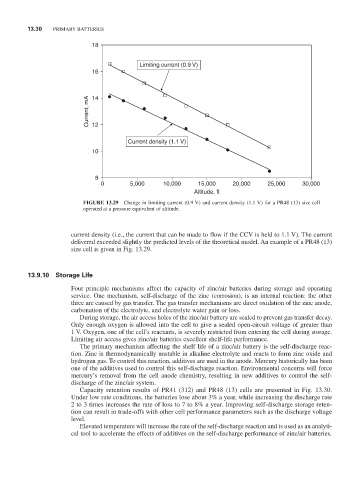
FiGURE 13.29 Change in limiting current (0.9 V) and current density (1.1 V) for a Pr48 (13) size cell
operated at a pressure equivalent of altitude.
current density (i.e., the current that can be made to flow if the CCV is held to 1.1 V). The current
delivered exceeded slightly the predicted levels of the theoretical model. An example of a Pr48 (13)
size cell is given in Fig. 13.29.
13.9.10 storage life
Four principle mechanisms affect the capacity of zinc/air batteries during storage and operating
service. One mechanism, self-discharge of the zinc (corrosion), is an internal reaction: the other
three are caused by gas transfer. The gas transfer mechanisms are direct oxidation of the zinc anode,
carbonation of the electrolyte, and electrolyte water gain or loss.
During storage, the air access holes of the zinc/air battery are sealed to prevent gas transfer decay.
Only enough oxygen is allowed into the cell to give a sealed open-circuit voltage of greater than
1 V. Oxygen, one of the cell’s reactants, is severely restricted from entering the cell during storage.
Limiting air access gives zinc/air batteries excellent shelf-life performance.
The primary mechanism affecting the shelf life of a zinc/air battery is the self-discharge reac-
tion. Zinc is thermodynamically unstable in alkaline electrolyte and reacts to form zinc oxide and
hydrogen gas. To control this reaction, additives are used in the anode. mercury historically has been
one of the additives used to control this self-discharge reaction. Environmental concerns will force
mercury’s removal from the cell anode chemistry, resulting in new additives to control the self-
discharge of the zinc/air system.
Capacity retention results of Pr41 (312) and Pr48 (13) cells are presented in Fig. 13.30.
Under low rate conditions, the batteries lose about 3% a year, while increasing the discharge rate
2 to 3 times increases the rate of loss to 7 to 8% a year. Improving self-discharge storage reten-
tion can result in trade-offs with other cell performance parameters such as the discharge voltage
level.
Elevated temperature will increase the rate of the self-discharge reaction and is used as an analyti-
cal tool to accelerate the effects of additives on the self-discharge performance of zinc/air batteries.