Page 325 - Lindens Handbook of Batteries
P. 325
BUTTOn CELL BATTErIES: SILVEr OxIDE–ZInC AnD ZInC-AIr SYSTEmS 13.31
100%
Percent capacity retention 90% PR41 (312) 1500 ohm PR41 (312) 620 ohm
80%
PR48 (13) 1500 ohm
PR48 (13) 374 ohm
70%
0 6 12 18 24
Months of storage
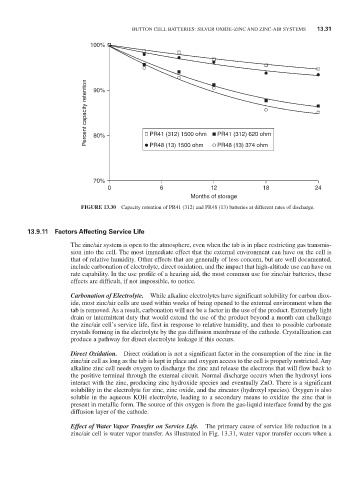
FiGURE 13.30 Capacity retention of Pr41 (312) and Pr48 (13) batteries at different rates of discharge.
13.9.11 Factors affecting service life
The zinc/air system is open to the atmosphere, even when the tab is in place restricting gas transmis-
sion into the cell. The most immediate effect that the external environment can have on the cell is
that of relative humidity. Other effects that are generally of less concern, but are well documented,
include carbonation of electrolyte, direct oxidation, and the impact that high-altitude use can have on
rate capability. In the use profile of a hearing aid, the most common use for zinc/air batteries, these
effects are difficult, if not impossible, to notice.
Carbonation of Electrolyte. While alkaline electrolytes have significant solubility for carbon diox-
ide, most zinc/air cells are used within weeks of being opened to the external environment when the
tab is removed. As a result, carbonation will not be a factor in the use of the product. Extremely light
drain or intermittent duty that would extend the use of the product beyond a month can challenge
the zinc/air cell’s service life, first in response to relative humidity, and then to possible carbonate
crystals forming in the electrolyte by the gas diffusion membrane of the cathode. Crystallization can
produce a pathway for direct electrolyte leakage if this occurs.
Direct Oxidation. Direct oxidation is not a significant factor in the consumption of the zinc in the
zinc/air cell as long as the tab is kept in place and oxygen access to the cell is properly restricted. Any
alkaline zinc cell needs oxygen to discharge the zinc and release the electrons that will flow back to
the positive terminal through the external circuit. normal discharge occurs when the hydroxyl ions
interact with the zinc, producing zinc hydroxide species and eventually ZnO. There is a significant
solubility in the electrolyte for zinc, zinc oxide, and the zincates (hydroxyl species). Oxygen is also
soluble in the aqueous KOH electrolyte, leading to a secondary means to oxidize the zinc that is
present in metallic form. The source of this oxygen is from the gas-liquid interface found by the gas
diffusion layer of the cathode.
Effect of Water Vapor Transfer on Service Life. The primary cause of service life reduction in a
zinc/air cell is water vapor transfer. As illustrated in Fig. 13.31, water vapor transfer occurs when a