Page 326 - Lindens Handbook of Batteries
P. 326
13.32 PrImArY BATTErIES
Humid day Dry day
O vapor to H O vapor from
H 2 2
electrolyte electrolyte
H 2 O vapor O 2 H 2 O vapor
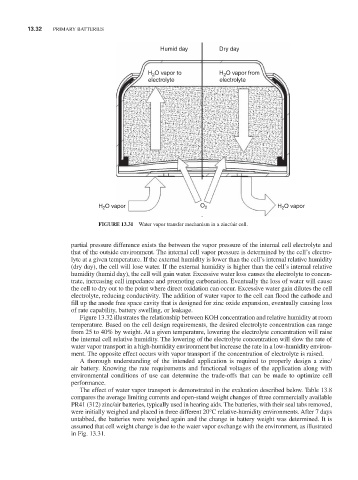
FiGURE 13.31 Water vapor transfer mechanism in a zinc/air cell.
partial pressure difference exists the between the vapor pressure of the internal cell electrolyte and
that of the outside environment. The internal cell vapor pressure is determined by the cell’s electro-
lyte at a given temperature. If the external humidity is lower than the cell’s internal relative humidity
(dry day), the cell will lose water. If the external humidity is higher than the cell’s internal relative
humidity (humid day), the cell will gain water. Excessive water loss causes the electrolyte to concen-
trate, increasing cell impedance and promoting carbonation. Eventually the loss of water will cause
the cell to dry out to the point where direct oxidation can occur. Excessive water gain dilutes the cell
electrolyte, reducing conductivity. The addition of water vapor to the cell can flood the cathode and
fill up the anode free space cavity that is designed for zinc oxide expansion, eventually causing loss
of rate capability, battery swelling, or leakage.
Figure 13.32 illustrates the relationship between KOH concentration and relative humidity at room
temperature. Based on the cell design requirements, the desired electrolyte concentration can range
from 25 to 40% by weight. At a given temperature, lowering the electrolyte concentration will raise
the internal cell relative humidity. The lowering of the electrolyte concentration will slow the rate of
water vapor transport in a high-humidity environment but increase the rate in a low-humidity environ-
ment. The opposite effect occurs with vapor transport if the concentration of electrolyte is raised.
A thorough understanding of the intended application is required to properly design a zinc/
air battery. Knowing the rate requirements and functional voltages of the application along with
environmental conditions of use can determine the trade-offs that can be made to optimize cell
performance.
The effect of water vapor transport is demonstrated in the evaluation described below. Table 13.8
compares the average limiting currents and open-stand weight changes of three commercially available
Pr41 (312) zinc/air batteries, typically used in hearing aids. The batteries, with their seal tabs removed,
were initially weighed and placed in three different 20°C relative-humidity environments. After 7 days
untabbed, the batteries were weighed again and the change in battery weight was determined. It is
assumed that cell weight change is due to the water vapor exchange with the environment, as illustrated
in Fig. 13.31.