Page 396 - Materials Science and Engineering An Introduction
P. 396
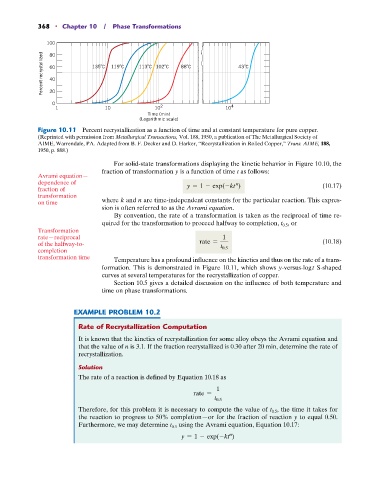
368 • Chapter 10 / Phase Transformations
100
Percent recrystallized 60 135 C 119 C 113 C 102 C 88 C 43 C
80
40
20
0
1 10 10 2 10 4
Time (min)
(Logarithmic scale)
Figure 10.11 Percent recrystallization as a function of time and at constant temperature for pure copper.
(Reprinted with permission from Metallurgical Transactions, Vol. 188, 1950, a publication of The Metallurgical Society of
AIME, Warrendale, PA. Adapted from B. F. Decker and D. Harker, “Recrystallization in Rolled Copper,” Trans. AIME, 188,
1950, p. 888.)
For solid-state transformations displaying the kinetic behavior in Figure 10.10, the
fraction of transformation y is a function of time t as follows:
Avrami equation—
dependence of n (10.17)
fraction of y = 1 - exp(-kt )
transformation
on time where k and n are time-independent constants for the particular reaction. This expres-
sion is often referred to as the Avrami equation.
By convention, the rate of a transformation is taken as the reciprocal of time re-
quired for the transformation to proceed halfway to completion, t 0.5 , or
Transformation
rate—reciprocal 1
of the halfway-to- rate = (10.18)
completion t 0.5
transformation time Temperature has a profound influence on the kinetics and thus on the rate of a trans-
formation. This is demonstrated in Figure 10.11, which shows y-versus-log t S-shaped
curves at several temperatures for the recrystallization of copper.
Section 10.5 gives a detailed discussion on the influence of both temperature and
time on phase transformations.
EXAMPLE PROBLEM 10.2
Rate of Recrystallization Computation
It is known that the kinetics of recrystallization for some alloy obeys the Avrami equation and
that the value of n is 3.1. If the fraction recrystallized is 0.30 after 20 min, determine the rate of
recrystallization.
Solution
The rate of a reaction is defined by Equation 10.18 as
1
rate =
t 0.5
Therefore, for this problem it is necessary to compute the value of t 0.5 , the time it takes for
the reaction to progress to 50% completion—or for the fraction of reaction y to equal 0.50.
Furthermore, we may determine t 0.5 using the Avrami equation, Equation 10.17:
n
y = 1 - exp(-kt )