Page 399 - Materials Science and Engineering An Introduction
P. 399
10.5 Isothermal Transformation Diagrams • 371
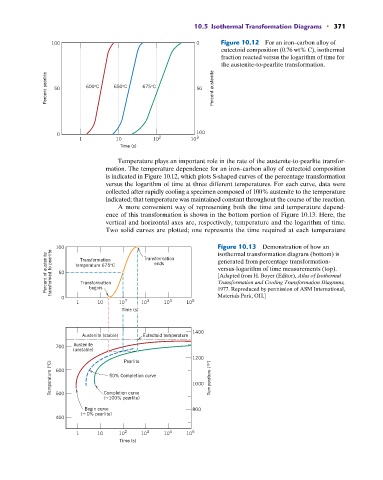
100 0 Figure 10.12 For an iron–carbon alloy of
eutectoid composition (0.76 wt% C), isothermal
fraction reacted versus the logarithm of time for
the austenite-to-pearlite transformation.
Percent pearlite 50 600°C 650°C 675°C 50 Percent austenite
100
0
1 10 10 2 10 3
Time (s)
Temperature plays an important role in the rate of the austenite-to-pearlite transfor-
mation. The temperature dependence for an iron–carbon alloy of eutectoid composition
is indicated in Figure 10.12, which plots S-shaped curves of the percentage transformation
versus the logarithm of time at three different temperatures. For each curve, data were
collected after rapidly cooling a specimen composed of 100% austenite to the temperature
indicated; that temperature was maintained constant throughout the course of the reaction.
A more convenient way of representing both the time and temperature depend-
ence of this transformation is shown in the bottom portion of Figure 10.13. Here, the
vertical and horizontal axes are, respectively, temperature and the logarithm of time.
Two solid curves are plotted; one represents the time required at each temperature
Figure 10.13 Demonstration of how an
Percent of austenite transformed to pearlite 100 temperature 675°C Transformation isothermal transformation diagram (bottom) is
generated from percentage transformation-
Transformation
ends
versus-logarithm of time measurements (top).
50
[Adapted from H. Boyer (Editor), Atlas of Isothermal
Transformation and Cooling Transformation Diagrams,
Transformation
Materials Park, OH.]
0 begins 1977. Reproduced by permission of ASM International,
1 10 10 2 10 3 10 4 10 5
Time (s)
1400
Austenite (stable) Eutectoid temperature
Austenite
700
(unstable)
1200
Pearlite
Temperature (°C) 600 50% Completion curve 1000 Temperature (°F)
Completion curve
500
(~100% pearlite)
Begin curve 800
(~0% pearlite)
400
1 10 10 2 10 3 10 4 10 5
Time (s)