Page 47 - Microsensors, MEMS and Smart Devices - Gardner Varadhan and Awadelkarim
P. 47
DOPING SEMICONDUCTORS 29
Electron pair (bound electrons) in a covalent bond
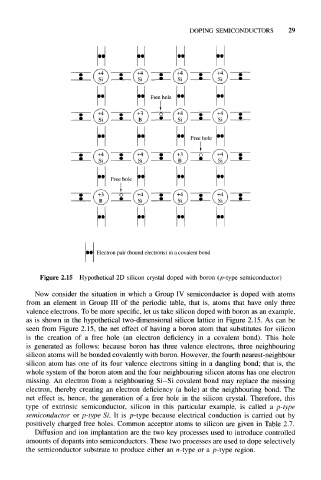
Figure 2.15 Hypothetical 2D silicon crystal doped with boron (p-type semiconductor)
Now consider the situation in which a Group IV semiconductor is doped with atoms
from an element in Group III of the periodic table, that is, atoms that have only three
valence electrons. To be more specific, let us take silicon doped with boron as an example,
as is shown in the hypothetical two-dimensional silicon lattice in Figure 2.15. As can be
seen from Figure 2.15, the net effect of having a boron atom that substitutes for silicon
is the creation of a free hole (an electron deficiency in a covalent bond). This hole
is generated as follows: because boron has three valence electrons, three neighbouring
silicon atoms will be bonded covalently with boron. However, the fourth nearest-neighbour
silicon atom has one of its four valence electrons sitting in a dangling bond; that is, the
whole system of the boron atom and the four neighbouring silicon atoms has one electron
missing. An electron from a neighbouring Si-Si covalent bond may replace the missing
electron, thereby creating an electron deficiency (a hole) at the neighbouring bond. The
net effect is, hence, the generation of a free hole in the silicon crystal. Therefore, this
type of extrinsic semiconductor, silicon in this particular example, is called a p-type
semiconductor or p-type Si. It is p-type because electrical conduction is carried out by
positively charged free holes. Common acceptor atoms to silicon are given in Table 2.7.
Diffusion and ion implantation are the two key processes used to introduce controlled
amounts of dopants into semiconductors. These two processes are used to dope selectively
the semiconductor substrate to produce either an n-type or a p-type region.