Page 72 - Microsensors, MEMS and Smart Devices - Gardner Varadhan and Awadelkarim
P. 72
SEMICONDUCTORS 53
semiconductor materials, from Group IV elements in the periodic table, are germanium
and carbon (diamond). Semiconductor materials can also be made from a combination of
elements either from Group III and Group V or from Group II and Group VI. Examples of
these are gallium arsenide and zinc telluride materials. The name semiconductor is given
to these materials because at certain regimes of temperatures they are able to exhibit
good electrical conduction properties, and outside these temperature regimes they behave
7
as insulators .
Semiconductor crystals can be made from both single elements and compounds. Semi-
conductors that are made from single elements are called elemental semiconductors.
Elemental semiconductors are found in Group IV of the periodic table, for example,
silicon (Si), and germanium (Ge). Compound semiconductors are made up of special
combinations either of Group III and Group V elements or of Group II and Group VI
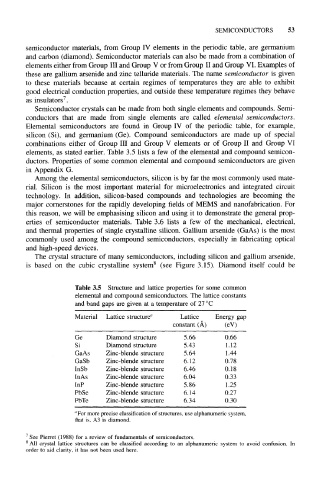
elements, as stated earlier. Table 3.5 lists a few of the elemental and compound semicon-
ductors. Properties of some common elemental and compound semiconductors are given
in Appendix G.
Among the elemental semiconductors, silicon is by far the most commonly used mate-
rial. Silicon is the most important material for microelectronics and integrated circuit
technology. In addition, silicon-based compounds and technologies are becoming the
major cornerstones for the rapidly developing fields of MEMS and nanofabrication. For
this reason, we will be emphasising silicon and using it to demonstrate the general prop-
erties of semiconductor materials. Table 3.6 lists a few of the mechanical, electrical,
and thermal properties of single crystalline silicon. Gallium arsenide (GaAs) is the most
commonly used among the compound semiconductors, especially in fabricating optical
and high-speed devices.
The crystal structure of many semiconductors, including silicon and gallium arsenide,
is based on the cubic crystalline system 8 (see Figure 3.15). Diamond itself could be
Table 3.5 Structure and lattice properties for some common
elemental and compound semiconductors. The lattice constants
and band gaps are given at a temperature of 27°C
Material Lattice structure a Lattice Energy gap
constant (A) (eV)
Ge Diamond structure 5.66 0.66
Si Diamond structure 5.43 1.12
GaAs Zinc-blende structure 5.64 1.44
GaSb Zinc-blende structure 6.12 0.78
InSb Zinc-blende structure 6.46 0.18
InAs Zinc-blende structure 6.04 0.33
InP Zinc-blende structure 5.86 1.25
PbSe Zinc-blende structure 6.14 0.27
PbTe Zinc-blende structure 6.34 0.30
"For more precise classification of structures, use alphanumeric system,
that is, A3 is diamond.
7
See Pierret (1988) for a review of fundamentals of semiconductors.
8
All crystal lattice structures can be classified according to an alphanumeric system to avoid confusion. In
order to aid clarity, it has not been used here.