Page 136 - Modern physical chemistry
P. 136
6.10 Variation of Vapor Pressure with Concentration 127
holds asymptotically asX B ~ 0 (XA ~ 1), This is known as Henry's law. Similarly for con-
stituent A, we have
[6.57]
as
XA ~0(XB~1).
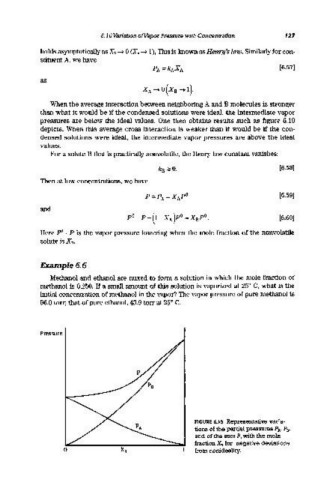
When the average interaction between neighboring A and B molecules is stronger
than what it would be if the condensed solutions were ideal, the intermediate vapor
pressures are below the ideal values. One then obtains results such as figure 6.19
depicts. When this average cross interaction is weaker than it would be if the con-
densed solutions were ideal, the intermediate vapor pressures are above the ideal
values.
For a solute B that is practically nonvolatile, the Henry law constant vanishes:
kB ~O. [6.58]
Then at low concentrations, we have
[6.59]
and
[6.60]
Here Ji! - P is the vapor pressure lowering when the mole fraction of the nonvolatile
solute is X B •
Example 6.6
Methanol and ethanol are mixed to form a solution in which the mole fraction of
methanol is 0.250. If a small amount of this solution is vaporized at 25 C, what is the
0
initial concentration of methanol in the vapor? The vapor pressure of pure methanol is
96.0 torr; that of pure ethanol, 43.9 torr at 25 C.
0
Pressure
FIGURE 6.19 Representative varia-
tions of the partial pressures PAl PBI
and of the sum P, with the mole
fraction X B for negative deviations
from nonideality.