Page 140 - Modern physical chemistry
P. 140
6.14 Depression of the Freezing Point 131
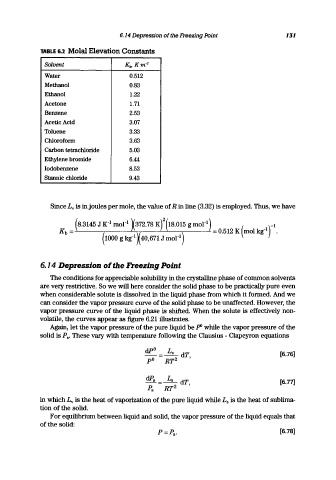
TABLE 6.2 Molal Elevation Constants
Solvent Kb,Km- 1
Water 0.512
Methanol 0.83
Ethanol 1.22
Acetone 1.71
Benzene 2.53
Acetic Acid 3.07
Toluene 3.33
Chlorofonn 3.63
Carbon tetrachloride 5.03
Ethylene bromide 6.44
Iodobenzene 8.53
Stannic chloride 9.43
Since Lv is in joules per mole, the value of R in line (3.32) is employed. Thus, we have
1
1
1
(S.3145JK- mOI- X372.7S Kt(I8.015 gmOI- ) ( )-1
Kb = = 0.512 K mol kg- 1
1
1
(1000 g kg- X 40,671 J mol- )
6. 14 Depression of the Freezing Point
The conditions for appreciable solubility in the crystalline phase of common solvents
are very restrictive. So we will here consider the solid phase to be practically pure even
when considerable solute is dissolved in the liquid phase from which it formed. And we
can consider the vapor pressure curve of the solid phase to be unaffected. However, the
vapor pressure curve of the liquid phase is shifted. When the solute is effectively non-
volatile, the curves appear as figure 6.21 illustrates.
Again, let the vapor pressure of the pure liquid be j:Jl while the vapor pressure of the
solid is Ps• These vary with temperature following the Clausius - Clapeyron equations
[6.76]
dP s =...b.- dT, [6.77]
P s RT2
in which Lv is the heat of vaporization of the pure liquid while Ls is the heat of sublima-
tion of the solid.
For equilibrium between liquid and solid, the vapor pressure of the liquid equals that
of the solid:
[6.78]