Page 165 - Book Hosokawa Nanoparticle Technology Handbook
P. 165
3.5 INTERACTIONS BETWEEN PARTICLES FUNDAMENTALS
The repulsive potential V between similar particles
Surface potential R
0 is given explicitly by the following equations, when
the magnitude of is sufficiently low.
Stern potential 0
d
Zeta potential a ln ⎡ a) ⎤
2
V 2 ⎣ 1 exp (r 2 ⎦ ( a 10)
R 0
(3.5.50)
(h)
2
(
r
h 4 a exp r 2a) ( a 5) (3.5.51)
0
where the sign in equation (3.5.50) indicates the
constant surface potential, and the – sign indicates the
constant surface charge. At a 5, V is given by
R
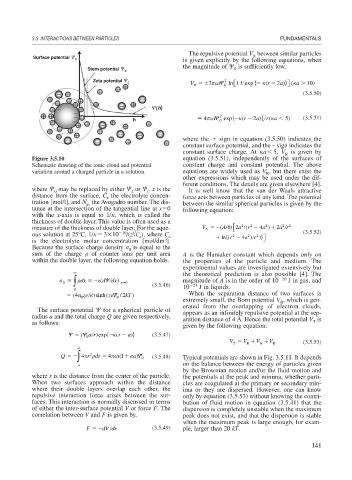
Figure 3.5.10 equation (3.5.51), independently of the surfaces of
Schematic drawing of the ionic cloud and potential constant charge and constant potential. The above
variation around a charged particle in a solution. equations are widely used as V , but there exist the
R
other expressions which may be used under the dif-
ferent conditions. The details are given elsewhere [4].
where may be replaced by either or . x is the It is well know that the van der Waals attractive
0
d
distance from the surface, C the electrolyte concen- force acts between particles of any kind. The potential
e
tration [mol/l], and N the Avogadro number. The dis- between the similar spherical particles is given by the
av
tance at the intersection of the tangential line at x 0 following equation:
with the x-axis is equal to 1/ , which is called the
thickness of double layer. This value is often used as a
2
2
2
2
measure of the thickness of double layer. For the aque- V ( A )62 ⎡ ⎣ a ( r 4 a ) 2 a r 2
A
ous solution at 25 C, 1/ 3 10 10 /(z C ), where C e ln ( r 4 a ) r ) ⎤ ⎦ (3.5.52)
2
2
2
e
3
is the electrolyte molar concentration [mol/dm ].
Because the surface charge density is equal to the
0
sum of the charge of counter ions per unit area A is the Hamaker constant which depends only on
within the double layer, the following equation holds. the properties of the particle and medium. The
experimental values are investigated extensively but
the theoretical prediction is also possible [4]. The
∫ dx d dx) x 0 magnitude of A is in the order of 10 20 J in gas, and
(
0
0 (3.5.46) 10 21 J in liquids.
( 4nze )sinh( ze / 2kT) When the separation distance of two surfaces is
0 0
extremely small, the Born potential V , which is gen-
B
erated from the overlapping of electron clouds,
The surface potential for a spherical particle of appears as an infinitely repulsive potential at the sep-
radius a and the total charge Q are given respectively, aration distance of 4 Å. Hence the total potential V is
´
as follows: T
given by the following equation:
( ar )exp (r ) (3.5.47)
a
0
V V V V (3.5.53)
T R A B
∫
r dr 4
Q 4 a 1 a) 0 (3.5.48) Typical potentials are shown in Fig. 3.5.11. It depends
2
(
a on the balance between the energy of particles given
by the Brownian motion and/or the fluid motion and
where r is the distance from the center of the particle. the potentials at the peak and minima, whether parti-
When two surfaces approach within the distance cles are coagulated at the primary or secondary min-
where their double layers overlap each other, the ima or they are dispersed. However, one can know
repulsive interaction force arises between the sur- only by equation (3.5.53) without knowing the contri-
faces. This interaction is normally discussed in terms bution of fluid motion in equation (3.5.41) that the
of either the inter-surface potential V or force F. The dispersion is completely unstable when the maximum
correlation between V and F is given by, peak does not exist, and that the dispersion is stable
when the maximum peak is large enough, for exam-
F dV dr (3.5.49) ple, larger than 20 kT.
141