Page 168 - Book Hosokawa Nanoparticle Technology Handbook
P. 168
FUNDAMENTALS CH. 3 CHARACTERISTICS AND BEHAVIOR OF NANOPARTICLES AND ITS DISPERSION SYSTEMS
Contact time of become stable because of the inter-particle repulsive
two surfaces t (s) Li + force due to the charge of the adsorbed surfactants.
c
6
Adhesive energy F ad /a (mN/m) 4 2 1 M + t = 0.1s + Na + If the particle surface is adsorbed completely in this
When surfactants with the charge opposite to the
t = 50 s
c
particle surface are dosed, the head groups are
adsorbed on the surface by the electrostatic attraction.
c
case, the surface becomes hydrophobic and particles
will be unstable by the hydrophobic attractive force.
When the surfactant is dosed further, the secondary
adsorbed layer will be formed such that the polar
heads are directed toward the solution. Then the sur-
K
Cs
stable again.
0
250 350 450 550 face charge is reversed and the suspension becomes
(iii) Non-DLVO interactions by polymers
Hydration enthalpy - H (kJ/mol)
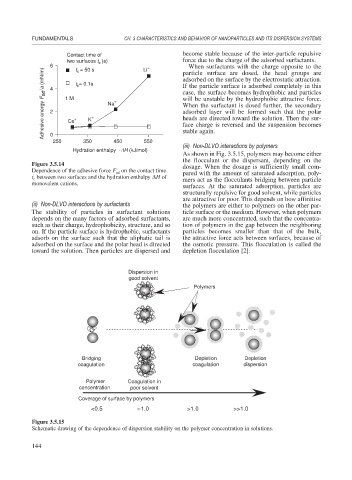
As shown in Fig. 3.5.15, polymers may become either
the flocculant or the dispersant, depending on the
Figure 3.5.14 dosage. When the dosage is sufficiently small com-
Dependence of the adhesive force F on the contact time pared with the amount of saturated adsorption, poly-
ad
t between two surfaces and the hydration enthalpy H of mers act as the flocculants bridging between particle
c
monovalent cations.
surfaces. At the saturated adsorption, particles are
structurally repulsive for good solvent, while particles
are attractive for poor. This depends on how affinitive
(ii) Non-DLVO interactions by surfactants the polymers are either to polymers on the other par-
The stability of particles in surfactant solutions ticle surface or the medium. However, when polymers
depends on the many factors of adsorbed surfactants, are much more concentrated, such that the concentra-
such as their charge, hydrophobicity, structure, and so tion of polymers in the gap between the neighboring
on. If the particle surface is hydrophobic, surfactants particles becomes smaller than that of the bulk,
adsorb on the surface such that the aliphatic tail is the attractive force acts between surfaces, because of
adsorbed on the surface and the polar head is directed the osmotic pressure. This flocculation is called the
toward the solution. Then particles are dispersed and depletion flocculation [2].
Dispersion in
good solvent
Polymers
Bridging Depletion Depletion
coagulation coagulation dispersion
Polymer Coagulation in
concentration poor solvent
Coverage of surface by polymers
<0.5 1.0 >1.0 >>1.0
Figure 3.5.15
Schematic drawing of the dependence of dispersion stability on the polymer concentration in solutions.
144