Page 170 - Book Hosokawa Nanoparticle Technology Handbook
P. 170
FUNDAMENTALS CH. 3 CHARACTERISTICS AND BEHAVIOR OF NANOPARTICLES AND ITS DISPERSION SYSTEMS
0
-50
5
Interaction potential F/a (mN/m) -100 -10 350 400 450
0
-5
-150
-200
approach (1st cycle)
separation (1st cycle)
-250 approach (6th cycle)
separation (6th cycle)
-300
0 100 200 300 400 500
Separation distance between particle surfaces h (nm)
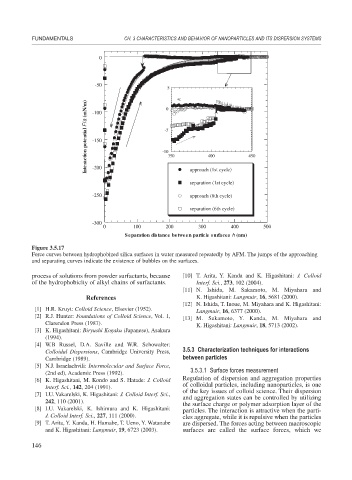
Figure 3.5.17
Force curves between hydrophobized silica surfaces in water measured repeatedly by AFM. The jumps of the approaching
and separating curves indicate the existence of bubbles on the surfaces.
process of solutions from powder surfactants, because [10] T. Arita, Y. Kanda and K. Higashitani: J. Colloid
of the hydrophobicity of alkyl chains of surfactants. Interf. Sci., 273, 102 (2004).
[11] N. Ishida, M. Sakamoto, M. Miyahara and
References K. Higashitani: Langmuir, 16, 5681 (2000).
[12] N. Ishida, T. Inoue, M. Miyahara and K. Higashitani:
[1] H.R. Kruyt: Colloid Science, Elsevier (1952). Langmuir, 16, 6377 (2000).
[2] R.J. Hunter: Foundations of Colloid Science, Vol. 1, [13] M. Sakamoto, Y. Kanda, M. Miyahara and
Clarendon Press (1987). K. Higashitani: Langmuir, 18, 5713 (2002).
[3] K. Higashitani: Biryushi Kogaku (Japanese), Asakura
(1994).
[4] W.B Russel, D.A. Saville and W.R. Schowalter:
3.5.3 Characterization techniques for interactions
Colloidal Dispersions, Cambridge University Press,
Cambridge (1989). between particles
[5] N.J. Israelachvili: Intermolecular and Surface Force,
3.5.3.1 Surface forces measurement
(2nd ed), Academic Press (1992).
Regulation of dispersion and aggregation properties
[6] K. Higashitani, M. Kondo and S. Hatade: J. Colloid
of colloidal particles, including nanoparticles, is one
Interf. Sci., 142, 204 (1991).
of the key issues of colloid science. Their dispersion
[7] I.U. Vakarelski, K. Higashitani: J. Colloid Interf. Sci.,
and aggregation states can be controlled by utilizing
242, 110 (2001).
the surface charge or polymer adsorption layer of the
[8] I.U. Vakarelski, K. Ishimura and K. Higashitani: particles. The interaction is attractive when the parti-
J. Colloid Interf. Sci., 227, 111 (2000). cles aggregate, while it is repulsive when the particles
[9] T. Arita, Y. Kanda, H. Hamabe, T. Ueno, Y. Watanabe are dispersed. The forces acting between macroscopic
and K. Higashitani: Langmuir, 19, 6723 (2003). surfaces are called the surface forces, which we
146