Page 167 - Book Hosokawa Nanoparticle Technology Handbook
P. 167
3.5 INTERACTIONS BETWEEN PARTICLES FUNDAMENTALS
small value of , the repulsive force is reduced and theory. This is caused by the structured force
0
particles will be coagulated. The control of 0 generated by the adsorbed layers of hydrated Li ,
depends on the charging mechanism. For example, which is sometimes called as the solvation force. The
the value of is changed by the solution pH for thickness of adsorbed layer follows the order of
0
oxide particles. It is known that the dispersion is hydration enthalpy of ions, Cs K Na Li and
2
2
unstable at 20 mV. Ca Mg , and the degree of coagulation of parti-
0
cles also follows this order.
3.5.2.4 Non-DLVO interactions These experimental results are very important
The non-DLVO interactions include all the interac- because the salvation forces do influence the stability
tions which cannot be explained by the DLVO of nanoparticles.
theory. The non-DLVO interaction appears for the
surfaces with adsorbed layers of water molecules, 1. When the particle size becomes smaller than
ions, hydrated ions, surfactants, polymers and 100 nm, the thickness of adsorbed layer is of the
nanobubbles. same order with the distance where the van der
Waals attraction influences. Hence, the proba-
(i) Non-DLVO interactions in solutions and the relation
bility of coagulation by the collision of particles
with the stability of nanoparticles
reduces exponentially, as shown in Fig. 3.5.13.
Not only polarized water molecules but also ions and
hydrated ions, more or less, adsorb on the charged This implies that the dispersion becomes more
surface of particles in solutions. The thickness of stable as the particle size decreases, if all the
these adsorbed layers depends on the properties of surface properties are the same [6].
particles and the medium. For silica and mica sur- 2. Because the strength of the van der Waals attrac-
faces, there exists the layer of ca. 1 nm thickness, that
is, the thickness of two or three layers of water mole- tion changes greatly at the small separation
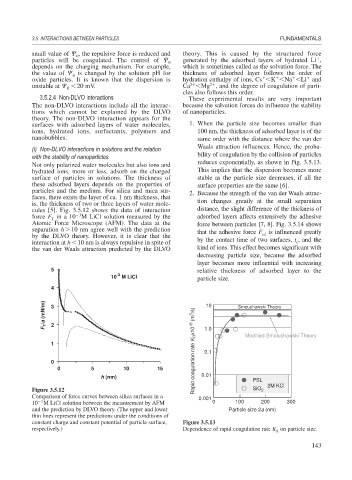
cules [5]. Fig. 3.5.12 shows the data of interaction distance, the slight difference of the thickness of
3
force F in a 10 M LiCl solution measured by the adsorbed layers affects extensively the adhesive
T
Atomic Force Microscope (AFM). The data at the force between particles [7, 8]. Fig. 3.5.14 shows
separation h 10 nm agree well with the prediction that the adhesive force F is influenced greatly
by the DLVO theory. However, it is clear that the ad
interaction at h 10 nm is always repulsive in spite of by the contact time of two surfaces, t , and the
c
the van der Waals attraction predicted by the DLVO kind of ions. This effect becomes significant with
decreasing particle size, because the adsorbed
layer becomes more influential with increasing
5 relative thickness of adsorbed layer to the
-3
10 M LiCl particle size.
4
F T /a (mN/m) 3 1.0 Smouchowski Theory
10
2
1 Rapid coagulation rate K R x10 18 (m 3 /s) 0.1 Modified Smoluchowski Theory
0
0 5 10 15
h (nm) 0.01 PSL
Figure 3.5.12 SiO 2 2M KCl
Comparison of force curves between silica surfaces in a 0.001
3
10 M LiCl solution between the measurement by AFM 0 100 200 300
and the prediction by DLVO theory. (The upper and lower Particle size 2a (nm)
thin lines represent the predictions under the conditions of
constant charge and constant potential of particle surface, Figure 3.5.13
respectively.) Dependence of rapid coagulation rate K on particle size.
R
143