Page 466 - Book Hosokawa Nanoparticle Technology Handbook
P. 466
APPLICATIONS 5 A DYE-SENSITIZED SOLAR CELL UTILIZING METAL NANOPARTICLE
0.5 0.5
Varying particle diameters and covered
0.4 (d) 0.4 surface ratios of the Ag island films
Absorbance (−) 0.3 (b) Absorbance (−) 0.3
(c)
0.2
0.2
0.1 (a) 0.1
0 0 0 1 10 -9 2 10 -9 3 10 -9 4 10 -9
300 400 500 600 700 800
2
Ru-dye concentration on the glass substrate (mol/cm )
Wavelength (nm)
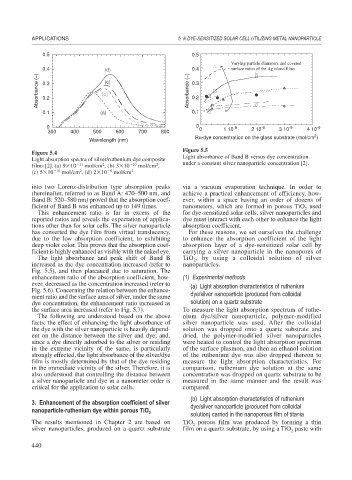
Figure 5.5
Figure 5.4
Light absorption spectra of silver/ruthenium dye composite Light absorbance of Band B versus dye concentration
2
2
films [2]. (a) 9 10 11 mol/cm , (b) 3 10 10 mol/cm , under a constant silver nanoparticle concentration [2].
2
2
(c) 5 10 10 mol/cm , (d) 2 10 9 mol/cm .
into two Lorenz-distribution type absorption peaks via a vacuum evaporation technique. In order to
(hereinafter, referred to as Band A: 470–500 nm, and achieve a practical enhancement of efficiency, how-
Band B: 520–580 nm) proved that the absorption coef- ever, within a space having an order of dozens of
ficient of Band B was enhanced up to 149 times. nanometers, which are formed in porous TiO used
2
This enhancement ratio is far in excess of the for dye-sensitized solar cells, silver nanoparticles and
reported ratios and reveals the expectation of applica- dye must interact with each other to enhance the light
tions other than for solar cells. The silver nanoparticle absorption coefficient.
has converted the dye film from virtual translucency, For these reasons, we set ourselves the challenge
due to the low absorption coefficient, to exhibiting to enhance the absorption coefficient of the light
deep violet color. This proves that the absorption coef- absorption layer of a dye-sensitized solar cell by
ficient is highly enhanced as visible with the naked eye. carrying a silver nanoparticle in the nanopores of
The light absorbance and peak shift of Band B TiO , by using a colloidal solution of silver
2
increased as the dye concentration increased (refer to nanoparticles.
Fig. 5.5), and then plateaued due to saturation. The
enhancement ratio of the absorption coefficient, how- (1) Experimental methods
ever, decreased as the concentration increased (refer to (a) Light absorption characteristics of ruthenium
Fig. 5.6). Concerning the relation between the enhance-
ment ratio and the surface area of silver, under the same dye/silver nanoparticle (produced from colloidal
dye concentration, the enhancement ratio increased as solution) on a quartz substrate
the surface area increased (refer to Fig. 5.7). To measure the light absorption spectrum of ruthe-
The following are understood based on the above nium dye/silver nanoparticle, polymer-modified
facts: the effect of enhancing the light absorbance of silver nanoparticle was used. After the colloidal
the dye with the silver nanoparticle is heavily depend- solution was dropped onto a quartz substrate and
ent on the distance between the silver and dye; and, dried, the polymer-modified silver nanoparticles
since a dye directly adsorbed to the silver or residing were heated to control the light absorption spectrum
in the extreme vicinity of the same, is particularly of the surface plasmon, and then an ethanol solution
strongly effected, the light absorbance of the silver/dye of the ruthenium dye was also dropped thereon to
film is mostly determined by that of the dye residing measure the light absorption characteristics. For
in the immediate vicinity of the silver. Therefore, it is comparison, ruthenium dye solution at the same
also understood that controlling the distance between concentration was dropped on quartz substrate to be
a silver nanoparticle and dye in a nanometer order is measured in the same manner and the result was
critical for the application to solar cells. compared.
(b) Light absorption characteristics of ruthenium
3. Enhancement of the absorption coefficient of silver
dye/silver nanoparticle (produced from colloidal
nanoparticle-ruthenium dye within porous TiO
2 solution) carried in the nanoporous film of titania
The results mentioned in Chapter 2 are based on TiO porous film was produced by forming a thin
2
silver nanoparticles, produced on a quartz substrate film on a quartz substrate, by using a TiO paste with
2
440