Page 465 - Book Hosokawa Nanoparticle Technology Handbook
P. 465
5 A DYE-SENSITIZED SOLAR CELL UTILIZING METAL NANOPARTICLE APPLICATIONS
If the absorbance of the dye via the local electric field
enhancement effect is enhanced, caused by a metal
nanoparticle, and if this enhanced result is utilized to pro-
duce a dye-sensitized solar cell, enhanced efficiency will
be expected. However, the magnitude of the absorbance
enhancement is heavily dependent on the combination of
a metal nanoparticle, semiconductor, and dye.
Therefore, we have set ourselves the challenge of
enhancing the absorption coefficient of the ruthenium
dye (cis-(NCS) bis(2,2 -bipyridyl-4,4 -dicarboxylate)
2
ruthenium(II) dye) with a silver nanoparticle, the
ruthenium dye reportedly high efficiency by about
10% in a DSC.
Fig. 5.3 exhibits the molecular structure of the
ruthenium dye (cis-(NCS) bis(2,2 -bipyridyl-4,4 -
2
dicarboxylate) ruthenium(II) dye) and the light
absorption spectrum. The ruthenium dye used has
absorptions at 400 and 535 nm, due to metal–ligand
charge transfer. Due to these absorptions, the dye-
sensitized solar cell generates a carrier.
Fig. 5.4 exhibits the absorption spectra of four
kinds of silver/dye films of variable dye concentra-
tions. This shows that the absorption at around
550 nm increases as the dye concentration increases.
Furthermore, although the concentration of the dye
film exhibited in Fig. 5.3 was higher than that of every
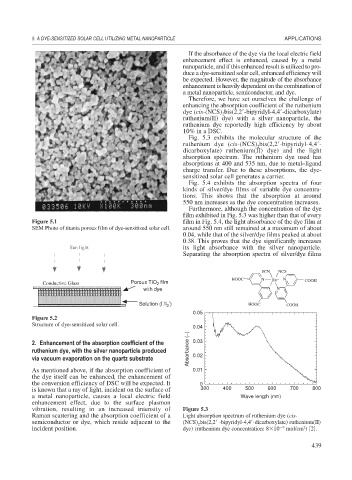
Figure 5.1 film in Fig. 5.4, the light absorbance of the dye film at
SEM Photo of titania porous film of dye-sensitized solar cell. around 550 nm still remained at a maximum of about
0.04, while that of the silver/dye films peaked at about
0.38. This proves that the dye significantly increases
Sun light its light absorbance with the silver nanoparticle.
Separating the absorption spectra of silver/dye films
SCN NCS
HOOC N Ru N COOH
Conductive Glass Porous TiO film
2
with dye N N
- -
Solution (I /I ) HOOC COOH
3
0.05
Figure 5.2
Structure of dye-sensitized solar cell. 0.04
Absorbance (−)
2. Enhancement of the absorption coefficient of the 0.03
ruthenium dye, with the silver nanoparticle produced 0.02
via vacuum evaporation on the quartz substrate
As mentioned above, if the absorption coefficient of 0.01
the dye itself can be enhanced, the enhancement of
the conversion efficiency of DSC will be expected. It 0
is known that a ray of light, incident on the surface of 300 400 500 600 700 800
a metal nanoparticle, causes a local electric field Wave length (nm)
enhancement effect, due to the surface plasmon
vibration, resulting in an increased intensity of Figure 5.3
Raman scattering and the absorption coefficient of a Light absorption spectrum of ruthenium dye (cis-
semiconductor or dye, which reside adjacent to the (NCS) bis(2,2 -bipyridyl-4,4 -dicarboxylate) ruthenium(II)
2
2
incident position. dye) (ruthenium dye concentration: 8 10 9 mol/cm ) [2].
439