Page 149 - Photoreactive Organic Thin Films
P. 149
128 ZOUHEIR SEKKAT AND WOLFGANG KNOLL
photoisomerization-induced molecular movement depends strongly on the
molecular structure of the unit building blocks of the polymer, a feature
confirmed in azo-polyurethanes.
4.4.2 Photoisomerization and Photo-Orientation of Flexible Azo-Polyurethanes
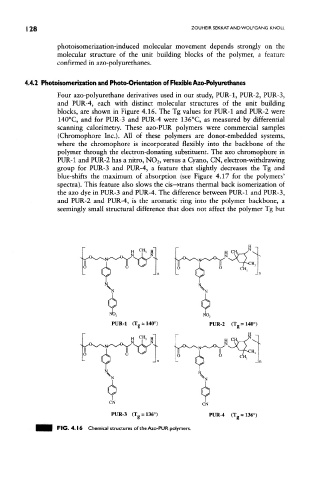
Four azo-polyurethane derivatives used in our study, PUR-1, PUR-2, PUR-3,
and PUR-4, each with distinct molecular structures of the unit building
blocks, are shown in Figure 4.16. The Tg values for PUR-1 and PUR-2 were
140°C, and for PUR-3 and PUR-4 were 136°C, as measured by differential
scanning calorimetry. These azo-PUR polymers were commercial samples
(Chromophore Inc.). All of these polymers are donor-embedded systems,
where the chromophore is incorporated flexibly into the backbone of the
polymer through the electron-donating substituent. The azo chromophore in
PUR-1 and PUR-2 has a nitro, NO 2, versus a Cyano, CN, electron-withdrawing
group for PUR-3 and PUR-4, a feature that slightly decreases the Tg and
blue-shifts the maximum of absorption (see Figure 4.17 for the polymers'
spectra). This feature also slows the cis—Hrans thermal back isomerization of
the azo dye in PUR-3 and PUR-4. The difference between PUR-1 and PUR-3,
and PUR-2 and PUR-4, is the aromatic ring into the polymer backbone, a
seemingly small structural difference that does not affect the polymer Tg but
PUR-1 (T g = 140°) PUR-2 (T g = 140°)
CN
PUR-3 (T g = 136°) PUR-4 (T g = 136°)
FIG. 4.16 Chemical structures of the Azo-PUR polymers.