Page 175 - Photoreactive Organic Thin Films
P. 175
154 MIKHAIL V. KOZLOVSKY, LEV M. BLINOV, AND WOLFGANG HAASE
2,5
2,0
<D
| 1,5
•e
I ''°
0,5
200 300 400 500 600 300 400 500 600
(A) Wavelength, X, nm Wavelength, X, nm (B)
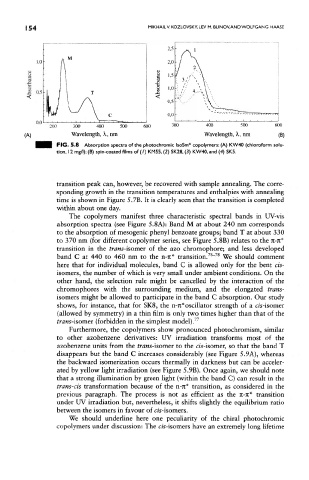
FIG. 5.8 Absorption spectra of the photochromic IsoSm* copolymers: (A) KW40 (chloroform solu-
tion, 1 2 mg/l); (B) spin-coated films of ( /) KM55, (2) SK28, (3) KW40, and (4) SK5.
transition peak can, however, be recovered with sample annealing. The corre-
sponding growth in the transition temperatures and enthalpies with annealing
time is shown in Figure 5.7B. It is clearly seen that the transition is completed
within about one day.
The copolymers manifest three characteristic spectral bands in UV-vis
absorption spectra (see Figure 5.8A): Band M at about 240 nm corresponds
to the absorption of mesogenic phenyl benzoate groups; band T at about 330
to 370 nm (for different copolymer series, see Figure 5.8B) relates to the n~n*
transition in the trans-isomer of the azo chromophore; and less developed
76 78
band C at 440 to 460 nm to the n-rc* transition. ' We should comment
here that for individual molecules, band C is allowed only for the bent cis-
isomers, the number of which is very small under ambient conditions. On the
other hand, the selection rule might be cancelled by the interaction of the
chromophores with the surrounding medium, and the elongated trans-
isomers might be allowed to participate in the band C absorption. Our study
shows, for instance, that for SK8, the n-n*oscillator strength of a o's-isorner
(allowed by symmetry) in a thin film is only two times higher than that of the
£ra«s-isomer (forbidden in the simplest model). 77
Furthermore, the copolymers show pronounced photochromism, similar
to other azobenzene derivatives: UV irradiation transforms most of the
azobenzene units from the trans-isomer to the ds-isomer, so that the band T
disappears but the band C increases considerably (see Figure 5.9A), whereas
the backward isomerization occurs thermally in darkness but can be acceler-
ated by yellow light irradiation (see Figure 5.9B). Once again, we should note
that a strong illumination by green light (within the band C) can result in the
tran$-ds transformation because of the n-n* transition, as considered in the
previous paragraph. The process is not as efficient as the n-n* transition
under UV irradiation but, nevertheless, it shifts slightly the equilibrium ratio
between the isomers in favour of m-isomers.
We should underline here one peculiarity of the chiral photochromic
copolymers under discussion: The cis-isomets have an extremely long lifetime