Page 96 - Physical chemistry eng
P. 96
4.4 THE TEMPERATURE DEPENDENCE OF REACTION ENTHALPIES 73
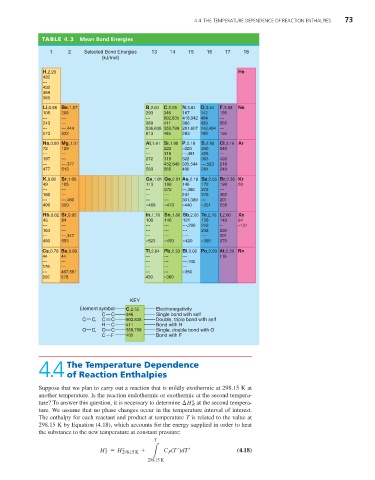
TABLE 4.3 Mean Bond Energies
1 2 Selected Bond Energies 13 14 15 16 17 18
(kJ/mol)
H,2.20 He
432
---
432
459
565
Li,0.98 Be,1.57 B,2.04 C,2.55 N,3.04 O,3.44 F,3.98 Ne
105 208 293 346 167 142 155
--- --- --- 602,835 418,942 494 ---
243 --- 389 411 386 459 565
--- ---,444 536,636 358,799 201,607 142,494 ---
573 632 613 485 283 190 155
Na,0.93 Mg,1.31 Al,1.61 Si,1.90 P,2.19 S,2.58 Cl,3.16 Ar
72 129 -- 222 ≈220 240 240
--- --- --- 318 ---,481 425 ---
197 --- 272 318 322 363 428
--- ---,377 --- 452,640 335,544 ---,523 218
477 513 583 565 490 284 249
K,0.82 Sr,1.00 Ga,1.81 Ge,2.01 As,2.18 Se,2.55 Br,2.96 Kr
49 105 113 188 146 172 190 50
--- --- --- 272 ---,380 272 ---
180 --- --- --- 247 276 362
--- ---,460 --- --- 301,389 --- 201
490 550 ≈469 ≈470 ≈440 ≈351 250
Rb,0.82 Sr,0.95 In,1.78 Sn,1.80 Sb,2.05 Te,2.10 I,2.66 Xe
45 84 100 146 121 126 149 84
--- --- --- --- ---,295 218 -- ≈131
163 --- --- --- --- 238 295
--- ---,347 --- --- ---- ---- 201
490 553 ≈523 ≈450 ≈420 ≈393 278
Cs,0.79 Ba,0.89 Tl,2.04 Pb,2.33 Bi,2.02 Po,2.00 At,2.20 Rn
44 44 --- --- --- 116
--- --- --- --- ---,192
176 --- --- --- ---
--- 467,561 --- --- ≈350
502 578 439 ≈360
KEY
Element symbol C,2.55 Electronegativity
C C 346 Single bond with self
C C, C C 602,835 Double, triple bond with self
H C 411 Bond with H
O C, O C 358,799 Single, double bond with O
C F 485 Bond with F
The Temperature Dependence
4.4 of Reaction Enthalpies
Suppose that we plan to carry out a reaction that is mildly exothermic at 298.15 K at
another temperature. Is the reaction endothermic or exothermic at the second tempera-
ture? To answer this question, it is necessary to determine ¢H° R at the second tempera-
ture. We assume that no phase changes occur in the temperature interval of interest.
The enthalpy for each reactant and product at temperature T is related to the value at
298.15 K by Equation (4.18), which accounts for the energy supplied in order to heat
the substance to the new temperature at constant pressure:
T
H° = H° 298.15 K + C (T¿)dT¿ (4.18)
P
T
298.15 K
3