Page 120 - Semiconductor Manufacturing Handbook
P. 120
Geng(SMH)_CH09.qxd 04/04/2005 19:42 Page 9.19
MICROLITHOGRAPHY
MICROLITHOGRAPHY 9.19
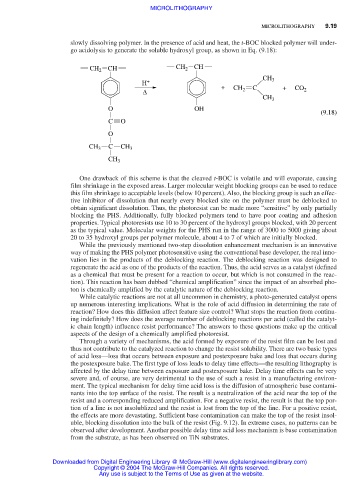
slowly dissolving polymer. In the presence of acid and heat, the t-BOC blocked polymer will under-
go acidolysis to generate the soluble hydroxyl group, as shown in Eq. (9.18):
CH 2 CH CH 2 CH
CH 3
H + +
∆ CH 2 C + CO 2
CH 3
O OH
(9.18)
C O
O
CH 3 C CH 3
CH 3
One drawback of this scheme is that the cleaved t-BOC is volatile and will evaporate, causing
film shrinkage in the exposed areas. Larger molecular weight blocking groups can be used to reduce
this film shrinkage to acceptable levels (below 10 percent). Also, the blocking group is such an effec-
tive inhibitor of dissolution that nearly every blocked site on the polymer must be deblocked to
obtain significant dissolution. Thus, the photoresist can be made more “sensitive” by only partially
blocking the PHS. Additionally, fully blocked polymers tend to have poor coating and adhesion
properties. Typical photoresists use 10 to 30 percent of the hydroxyl groups blocked, with 20 percent
as the typical value. Molecular weights for the PHS run in the range of 3000 to 5000 giving about
20 to 35 hydroxyl groups per polymer molecule, about 4 to 7 of which are initially blocked.
While the previously mentioned two-step dissolution enhancement mechanism is an innovative
way of making the PHS polymer photosensitive using the conventional base developer, the real inno-
vation lies in the products of the deblocking reaction. The deblocking reaction was designed to
regenerate the acid as one of the products of the reaction. Thus, the acid serves as a catalyst (defined
as a chemical that must be present for a reaction to occur, but which is not consumed in the reac-
tion). This reaction has been dubbed “chemical amplification” since the impact of an absorbed pho-
ton is chemically amplified by the catalytic nature of the deblocking reaction.
While catalytic reactions are not at all uncommon in chemistry, a photo-generated catalyst opens
up numerous interesting implications. What is the role of acid diffusion in determining the rate of
reaction? How does this diffusion affect feature size control? What stops the reaction from continu-
ing indefinitely? How does the average number of deblocking reactions per acid (called the catalyt-
ic chain length) influence resist performance? The answers to these questions make up the critical
aspects of the design of a chemically amplified photoresist.
Through a variety of mechanisms, the acid formed by exposure of the resist film can be lost and
thus not contribute to the catalyzed reaction to change the resist solubility. There are two basic types
of acid loss—loss that occurs between exposure and postexposure bake and loss that occurs during
the postexposure bake. The first type of loss leads to delay time effects—the resulting lithography is
affected by the delay time between exposure and postexposure bake. Delay time effects can be very
severe and, of course, are very detrimental to the use of such a resist in a manufacturing environ-
ment. The typical mechanism for delay time acid loss is the diffusion of atmospheric base contami-
nants into the top surface of the resist. The result is a neutralization of the acid near the top of the
resist and a corresponding reduced amplification. For a negative resist, the result is that the top por-
tion of a line is not insolublized and the resist is lost from the top of the line. For a positive resist,
the effects are more devastating. Sufficient base contamination can make the top of the resist insol-
uble, blocking dissolution into the bulk of the resist (Fig. 9.12). In extreme cases, no patterns can be
observed after development. Another possible delay time acid loss mechanism is base contamination
from the substrate, as has been observed on TiN substrates.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.