Page 55 - Semiconductor Manufacturing Handbook
P. 55
Geng(SMH)_CH05.qxd 04/04/2005 19:37 Page 5.4
FUNDAMENTALS OF SILICIDE FORMATION ON Si
5.4 SEMICONDUCTOR FUNDAMENTALS AND BASIC MATERIALS
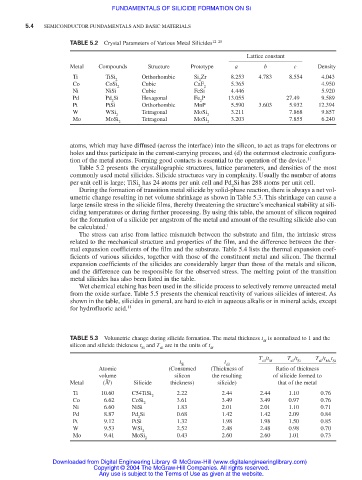
TABLE 5.2 Crystal Parameters of Various Metal Silicides 12–20
Lattice constant
Metal Compounds Structure Prototype a b c Density
Ti TiSi Orthorhombic Si Zr 8.253 4.783 8.554 4.043
2 2
Co CoSi Cubic CaF 5.365 4.950
2 2
Ni NiSi Cubic FeSi 4.446 5.920
Pd Pd Si Hexagonal Fe P 13.055 27.49 9.589
2 2
Pt PtSi Orthorhombic MnP 5.590 3.603 5.932 12.394
W WSi Tetragonal MoSi 3.211 7.868 9.857
2 2
Mo MoSi Tetragonal MoSi 3.203 7.855 6.240
2 2
atoms, which may have diffused (across the interface) into the silicon, to act as traps for electrons or
holes and thus participate in the current-carrying process, and (d) the outermost electronic configura-
tion of the metal atoms. Forming good contacts is essential to the operation of the device. 11
Table 5.2 presents the crystallographic structures, lattice parameters, and densities of the most
commonly used metal silicides. Silicide structures vary in complexity. Usually the number of atoms
per unit cell is large; TiSi has 24 atoms per unit cell and Pd Si has 288 atoms per unit cell.
2 2
During the formation of transition metal silicide by solid-phase reaction, there is always a net vol-
umetric change resulting in net volume shrinkage as shown in Table 5.3. This shrinkage can cause a
large tensile stress in the silicide films, thereby threatening the structure’s mechanical stability at sili-
ciding temperatures or during further processing. By using this table, the amount of silicon required
for the formation of a silicide per angstrom of the metal and amount of the resulting silicide also can
be calculated. 1
The stress can arise from lattice mismatch between the substrate and film, the intrinsic stress
related to the mechanical structure and properties of the film, and the difference between the ther-
mal expansion coefficients of the film and the substrate. Table 5.4 lists the thermal expansion coef-
ficients of various silicides, together with those of the constituent metal and silicon. The thermal
expansion coefficients of the silicides are considerably larger than those of the metals and silicon,
and the difference can be responsible for the observed stress. The melting point of the transition
metal silicides has also been listed in the table.
Wet chemical etching has been used in the silicide process to selectively remove unreacted metal
from the oxide surface. Table 5.5 presents the chemical reactivity of various silicides of interest. As
shown in the table, silicides in general, are hard to etch in aqueous alkalis or in mineral acids, except
for hydrofluoric acid. 11
TABLE 5.3 Volumetric change during silicide formation. The metal thickness t is normalized to 1 and the
M
silicon and silicide thickness t and T are in the units of t
Si sil M
T /t T /t T /t t
t t sil M sil Si sil M+ Si
Si sil
Atomic (Consumed (Thickness of Ratio of thickness
volume silicon the resulting of silicide formed to
°3
Metal (Α) Silicide thickness) silicide) that of the metal
Ti 10.60 C54TiSi 2.22 2.44 2.44 1.10 0.76
2
Co 6.62 CoSi 3.61 3.49 3.49 0.97 0.76
2
Ni 6.60 NiSi 1.83 2.01 2.01 1.10 0.71
Pd 8.87 Pd Si 0.68 1.42 1.42 2.09 0.84
2
Pt 9.12 PtSi 1.32 1.98 1.98 1.50 0.85
W 9.53 WSi 2.52 2.48 2.48 0.98 0.70
2
Mo 9.41 MoSi 0.43 2.60 2.60 1.01 0.73
2
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.