Page 56 - Semiconductor Manufacturing Handbook
P. 56
Geng(SMH)_CH05.qxd 04/04/2005 19:37 Page 5.5
FUNDAMENTALS OF SILICIDE FORMATION ON Si
FUNDAMENTALS OF SILICIDE FORMATION ON Si 5.5
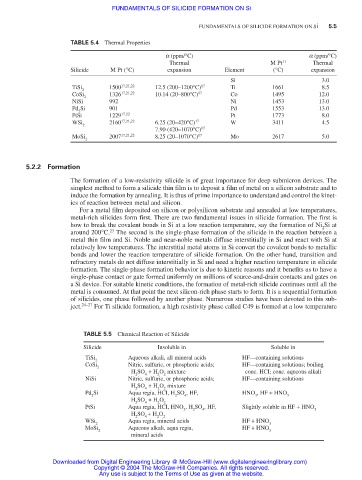
TABLE 5.4 Thermal Properties
a (ppm/°C) a (ppm/°C)
Thermal M Pt 11 Thermal
Silicide M Pt (°C) expansion Element (°C) expansion
Si 3.0
TiSi 1500 17,21,22 12.5 (200–1200°C) 17 Ti 1661 8.5
2
CoSi 1326 17,21,22 10.14 (20–800°C) 17 Co 1495 12.0
2
NiSi 992 Ni 1453 13.0
Pd Si 901 Pd 1553 13.0
2
PtSi 1229 17,22 Pt 1773 8.0
WSi 2160 17,21,22 6.25 (20–420°C) 17 W 3411 4.5
2
7.90 (420–1070°C) 17
MoSi 2007 17,21,22 8.25 (20–1070°C) 17 Mo 2617 5.0
2
5.2.2 Formation
The formation of a low-resistivity silicide is of great importance for deep submicron devices. The
simplest method to form a silicide thin film is to deposit a film of metal on a silicon substrate and to
induce the formation by annealing. It is thus of prime importance to understand and control the kinet-
ics of reaction between metal and silicon.
For a metal film deposited on silicon or polysilicon substrate and annealed at low temperatures,
metal-rich silicides form first. There are two fundamental issues in silicide formation. The first is
how to break the covalent bonds in Si at a low reaction temperature, say the formation of Ni Si at
2
23
around 200°C. The second is the single-phase formation of the silicide in the reaction between a
metal thin film and Si. Noble and near-noble metals diffuse interstitially in Si and react with Si at
relatively low temperatures. The interstitial metal atoms in Si convert the covalent bonds to metallic
bonds and lower the reaction temperature of silicide formation. On the other hand, transition and
refractory metals do not diffuse interstitially in Si and need a higher reaction temperature in silicide
formation. The single-phase formation behavior is due to kinetic reasons and it benefits us to have a
single-phase contact or gate formed uniformly on millions of source-and-drain contacts and gates on
a Si device. For suitable kinetic conditions, the formation of metal-rich silicide continues until all the
metal is consumed. At that point the next silicon-rich phase starts to form. It is a sequential formation
of silicides, one phase followed by another phase. Numerous studies have been devoted to this sub-
ject. 24–27 For Ti silicide formation, a high resistivity phase called C49 is formed at a low temperature
TABLE 5.5 Chemical Reaction of Silicide
Silicide Insoluble in Soluble in
TiSi Aqueous alkali, all mineral acids HF—containing solutions
2
CoSi Nitric, sulfuric, or phosphoric acids; HF—containing solutions; boiling
2
H SO + H O mixture conc. HCI; conc. aqueous alkali
2 4 2 2
NiSi Nitric, sulfuric, or phosphoric acids; HF—containing solutions
H SO + H O mixture
2 4 2 2
Pd Si Aqua regia, HCI, H SO , HF, HNO , HF + HNO
2 2 4 3 3
H SO + H O
2 4 2 2
PtSi Aqua regia, HCI, HNO , H SO , HF, Slightly soluble in HF + HNO
3 2 4 3
H SO + H O
2 4 2 2
WSi Aqua regia, mineral acids HF + HNO
2 3
MoSi Aqueous alkali, aqua regia, HF + HNO
2 3
mineral acids
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.