Page 137 - Separation process principles 2
P. 137
102 Chapter 3 Mass Transfer and Diffusion
for Prandtl numbers greater than 1 is Chilton-Colburn correlation, which is widely used, is within
10% of the more theoretically based Churchill-Zajic equa-
tion for Reynolds numbers up to 1,000,000. However, beyond
that, serious deviations occur (25% at NRe = 10,000,000 and
almost 50% at NRe = 100,000,000). Deviations of the
Friend-Metzner correlation from the Churchill-Zajic equa-
where, from Yu, Ozoe, and Churchill [72], tion vary from about 15% to 30% over the entire range of
Reynolds number in Table 3.15. At all Reynolds numbers,
0.015
(3-184)
Npr, = turbulent Prandtl number = 0.85 + - the Churchill-Zajic equation predicts higher Nusselt num-
Npr bers and, therefore, higher heat-transfer coefficients.
-
which replaces (u;T1), as introduced by Churchill 1741, At a Prandtl number of 1,000, which is typical of high-
viscosity liquids, the Friend-Metzner correlation is in fairly
NNu, = Nusselt number for (Npr = Nps)
close agreement with the Churchill-Zajic equation, predict-
ing values from 6 to 13% higher. The Chilton-Colburn cor-
relation is seriously in error over the entire range of
Reynolds number, predicting values ranging from 74 to 27%
of those from the Churchill-Zajic equation as the Reynolds
number increases. It is clear that the Chilton-Colburn corre-
NN~, lation should not be used at high Prandtl numbers for heat
= Nusselt number for (Npr = co)
transfer or (by analogy) at high Schmidt numbers for mass
transfer.
The Churchill-Zajic equation for predicting the Nusselt
The accuracy of (3-183) is due to (3-185) and (3-186), which number provides an effective power dependence on the
are known from theoretical considerations. Although (3-184) Reynolds number as the Reynolds number increases. This is
is somewhat uncertain, its effect is negligible. in contrast to the typically cited constant exponent of 0.8, as
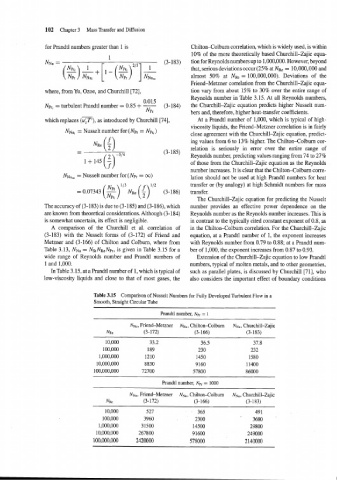
A comparison of the Churchill et al. correlation of in the Chilton-Colburn correlation. For the Churchill-Zajic
(3-183) with the Nusselt forms of (3-172) of Friend and equation, at a Prandtl number of 1, the exponent increases
Metzner and (3-166) of Chilton and Colburn, where from with Reynolds number from 0.79 to 0.88; at a Prandtl num-
Table 3.13, NNu = NStNRe NPr, is given in Table 3.15 for a ber of 1,000, the exponent increases from 0.87 to 0.93.
wide range of Reynolds number and Prandtl numbers of Extension of the Churchill-Zajic equation to low Prandtl
1 and 1,000. numbers, typical of molten metals, and to other geometries,
In Table 3.15, at a Prandtl number of 1, which is typical of such as parallel plates, is discussed by Churchill [71], who
low-viscosity liquids and close to that of most gases, the also considers the important effect of boundary conditions
Table 3.15 Comparison of Nusselt Numbers for Fully Developed Turbulent Flow in a
Smooth, Straight Circular Tube
Prandtl number, Npr = I
NN", Friend-Metzner NN,, , Chilton-Colburn NNu. Churchill-Zajic
NRe (3-172) (3- 166) (3-183)
Prandtl number, Npr = 1000
NN,,, Friend-Metzner NNu, Chilton-Colburn NNU, Churchill-Zajic
NRe (3-172) (3-166) (3-183)
10,000 527 365 49 1
100,000 3960 2300 ' 3680
1,000,000 31500 14500 29800
10,000,000 267800 9 1600 249000
100,000,000 2420000 578000 2 140000