Page 155 - Separation process principles 2
P. 155
120 Chapter 4 Single Equilibrium Stages and Flash Calculations
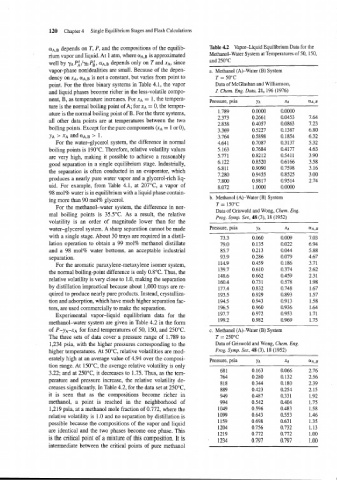
UA,B depends on T, P, and the compositions of the equilib- Table 4.2 Vapor-Liquid Equilibrium Data for the
rium vapor and liquid. At 1 atm, where %,B is approximated Methanol-Water System at Temperatures of 50, 150,
and 250°C
well by yAPL/yB Pi, UA,B depends only on T and XA, since
vapor-phase nonidealities are small. Because of the depen- a. Methanol (A)-Water (B) System
dency on xA, aA,B is not a constant, but varies from point to T = 50°C
point. For the three binary systems in Table 4.1, the vapor Data of McGlashan and Williamson,
and liquid phases become richer in the less-volatile compo- J. Chem. Eng. Data, 21, 196 (1976)
nent, B, as temperature increases. For XA = 1, the tempera- Pressure, psia YA XA ~A,B
ture is the normal boiling point of A; for xA = 0, the temper-
1.789 0.0000 0.0000
ature is the normal boiling point of B. For the three systems,
2.373 0.2661 0.0453
all other data points are at temperatures between the two
2.838 0.4057 0.0863
boiling points. Except for the pure components (XA = 1 or 0),
3.369 0.5227 0.1387
YA > xA and CYA,B > 1. 3.764 0.5898 0.1854
For the water-glycerol system, the difference in normal 4.641 0.7087 0.3137
boiling points is 190°C. Therefore, relative volatility values 5.163 0.7684 0.4177
are very high, making it possible to achieve a reasonably 5.771 0.8212 0.5411
6.122 0.8520 0.6166
good separation in a single equilibrium stage. Industrially,
6.811 0.9090 0.7598
the separation is often conducted in an evaporator, which
7.280 0.9455 0.8525
produces a nearly pure water vapor and a glycerol-rich liq-
7.800 0.9817 0.9514
uid. For example, from Table 4.1, at 207"C, a vapor of 8.072 1 .OOOO 0.0000
98 mol% water is in equilibrium with a liquid phase contain-
b. Methanol (A)-Water (B) System
ing more than 90 mol% glycerol.
T = 150°C
For the methanol-water system, the difference in nor-
Data of Griswold and Wong, Chem. Eng.
mal boiling points is 35.5"C. As a result, the relative
Prog. Symp. Ser, 48 (3), 18 (1952)
volatility is an order of magnitude lower than for the
water-glycerol system. A sharp separation cannot be made Pressure, psia YA XA ~A,B
with a single stage. About 30 trays are required in a distil-
lation operation to obtain a 99 mol% methanol distillate
and a 98 mol% water bottoms, an acceptable industrial
separation.
For the aromatic paraxylene-metaxylene isomer system,
the normal boiling-point difference is only 0.8"C. Thus, the
relative volatility is very close to 1.0, making the separation
by distillation impractical because about 1,000 trays are re-
quired to produce nearly pure products. Instead, crystalliza-
tion and adsorption, which have much higher separation fac-
tors, are used commercially to make the separation.
Experimental vapor-liquid equilibrium data for the
methanol-water system are given in Table 4.2 in the form
of P-yA-xA for fixed temperatures of 50, 150, and 250°C. c. Methanol (A)-Water (B) System
The three sets of data cover a pressure range of 1.789 to T = 250°C
1,234 psia, with the higher pressures corresponding to the Data of Griswold and Wong, Chem. Eng.
higher temperatures. At 50°C relative volatilities are mod- Prog. Symp. Ser, 48 (3), 18 (1952)
erately high at an average value of 4.94 over the composi- Pressure, psia YA XA ~A,B
tion range. At 150°C, the average relative volatility is only
3.22; and at 250°C, it decreases to 1.75. Thus, as the tem-
perature and pressure increase, the relative volatility de-
creases significantly. In Table 4.2, for the data set at 250°C,
it is seen that as the compositions become richer in
methanol, a point is reached in the neighborhood of
1,219 psia, at a methanol mole fraction of 0.772, where the
relative volatility is 1.0 and no separation by distillation is
possible because the compositions of the vapor and liquid
are identical and the two phases become one phase. This
is the critical point of a mixture of this composition. It is
intermediate between the critical points of pure methanol