Page 160 - Separation process principles 2
P. 160
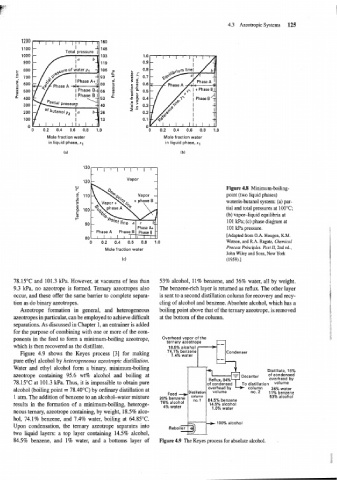
4.3 Azeotropic Systems 125
Mole fraction water Mole fraction water
in liquid phase, xl in liquid phase, xl
Vapor -
120 -
- Figure 4.8 Minimum-boiling-
point (two liquid phases)
waterln-butanol system: (a) par-
tial and total pressures at 100°C;
(b) vapor-liquid equilibria at
101 kPa; (c) phase diagram at
Phase At - 101 kPa pressure.
- Phase A Phase B Phase B
[Adapted from O.A. Hougen, K.M.
80 1 1 1 1 1 1 1 1 1 -
0 0.2 0.4 0.6 0.8 1.0 Watson, and R.A. Ragatz, Chemical
Process Principles. Part II, 2nd ed.,
Mole fraction water
John Wiley and Sons, New York
(c) (1959).]
78.15"C and 101.3 kPa. However, at vacuums of less than 53% alcohol, 11% benzene, and 36% water, all by weight.
9.3 kPa, no azeotrope is formed. Ternary azeotropes also The benzene-rich layer is returned as reflux. The other layer
occur, and these offer the same barrier to complete separa- is sent to a second distillation column for recovery and recy-
tion as do binary azeotropes. cling of alcohol and benzene. Absolute alcohol, which has a
Azeotrope formation in general, and heterogeneous boiling point above that of the ternary azeotrope, is removed
azeotropes in particular, can be employed to achieve difficult at the bottom of the column.
separations. As discussed in Chapter 1, an entrainer is added
for the purpose of combining with one or more of the com-
ponents in the feed to form a minimum-boiling azeotrope, Overhead vapor of the
ternary azeotrope
which is then recovered as the distillate. 18.5% alcohol
Figure 4.9 shows the Keyes process [3] for making 74.1% benzene
7.4% water
pure ethyl alcohol by heterogeneous azeotropic distillation.
Water and ethyl alcohol form a binary, minimum-boiling
Distillate, 16%
azeotrope containing 95.6 wt% alcohol and boiling at Decanter of condensed
overhead by
78.15"C at 101.3 kPa. Thus, it is impossible to obtain pure To distillation
alcohol (boiling point = 78.40°C) by ordinary distillation at overhead by L) column 36% water
no. 2 11% benzene
1 atm. The addition of benzene to an alcohol-water mixture 53% alcohol
results in the formation of a minimum-boiling, heteroge-
4% water 14.5% alcohol
1.0% water
neous ternary, azeotrope containing, by weight, 18.5% alco-
hol, 74.1% benzene, and 7.4% water, boiling at 64.85OC.
100% alcohol
Upon condensation, the ternary azeotrope separates into
two liquid layers: a top layer containing 14.5% alcohol,
84.5% benzene, and 1% water, and a bottoms layer of Figure 4.9 The Keyes process for absolute alcohol.