Page 159 - Separation process principles 2
P. 159
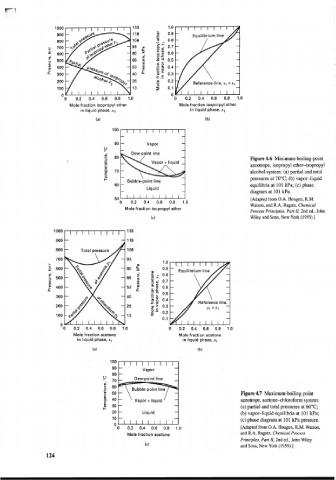
"0 0.2 0.4 0.6 0.8 1.0 0 0.2 0.4 0.6 0.8 1.0
Mole fraction isopropyl ether Mole fraction isopropyl ether
in liquid phase, xl in liquid phase, x1
Y
Dew-point line
Figure 4.6 Minimum-boiling-point
Vapor + liquid
azeotrope, isopropyl ether-isopropyl
a 70 alcohol system: (a) partial and total
60 t Liquid equilibria at 101 Wa; (c) phase
I- ~ubble-point line pressures at 70°C; (b) vapor-liquid
diagram at 101 Wa.
[Adapted from O.A. Hougen, K.M.
Watson, and R.A. Ragatz, Chemical
Mole fraction isopropyl ether
Process Principles. Part 11, 2nd ed., John
Wiley and Sons, New York (1959).]
Mole fraction acetone Mole fraction acetone
in liquid phase, xl in liquid phase, xl
I I I I I I I I I
- -
Vapor
- -
- Dew-point line -
- 60
2 50 Figure 4.7 Maximum-boiling-point
40 azeotrope, acetone-chloroform system:
k 30 - - (a) partial and total pressures at 60°C;
I- -
20 Liquid - (b) vapor-liquid equilibria at 101 kPa;
10 - - (c) phase diagram at 101 kPa pressure.
I I I I I I I I I
0
0 0.2 0.4 0.6 0.8 [Adapted from O.A. Hougen, K.M. Watson,
and R.A. Ragatz, Chemical Process
Mole fraction acetone
Principles. Part 11, 2nd ed., John Wiley
and Sons, New York (1959).]