Page 110 - Advances in Biomechanics and Tissue Regeneration
P. 110
106 6. REVIEW OF THE ESSENTIAL ROLES OF SMCS IN ATAA BIOMECHANICS
6.4 MECHANOSENSING AND MECHANOTRANSDUCTION
Given their highly sensitive cytoskeleton and FAs (Fig. 6.8), SMCs represent real sensors of the local mechanochem-
ical state of the ECM. Many experimental models have permitted the investigation of this mechanosensing role
and how it is involved in ATAAs and dissections [15]. One of the main responses to stimuli mechanosensing is
mechanotransduction [43, 124–127], which is the process of transducing wall stress stimuli into tissue remodeling
[21, 27, 32, 37, 42].
6.4.1 Mechanosensing
Many recent studies have highlighted the effects of the environment on the SMC response, in terms of protein syn-
thesis, proliferation, migration, differentiation, or apoptosis, thanks to its mechanosensitive architecture [43, 124–128].
Mechanosensing relies on links between the ECM, FAs, and the cytoskeleton. The microfibrils provide an adhesive
support to the SMCs through collagen VI [59]. Because of this, when microfibrils are damaged, SMCs sense an increase
in stiffness and are no longer able to transmit forces to each other through elastic fiber. According to several studies, the
elastin acts for the maturation of the contractile apparatus of the SMCs and may encourage their quiescent phenotype
[9, 41, 129]. In the ECM, two proteins are mainly involved in mechanosensing: fibronectin and laminin. Fibronectin is
known for being mainly present in the ECM of blood vessels during early development and seems to encourage SMC
proliferation and migration in order to build the tissue [34, 36]. On the contrary, the laminin may be required later for
SMC maturation toward a contractile phenotype [130].
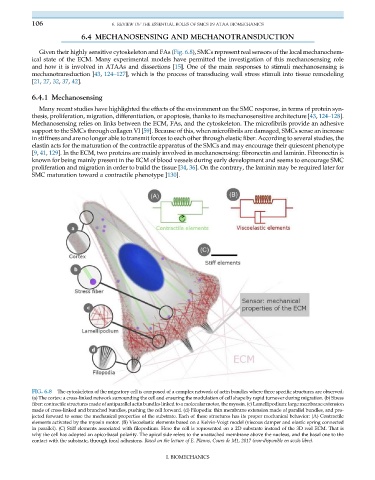
FIG. 6.8 The cytoskeleton of the migratory cell is composed of a complex network of actin bundles where three specific structures are observed:
(a) The cortex: a cross-linked network surrounding the cell and ensuring the modulation of cell shape by rapid turnover during migration. (b) Stress
fiber: contractile structures made of antiparallel actin bundles linked to a molecular motor, the myosin. (c) Lamellipodium: large membrane extension
made of cross-linked and branched bundles, pushing the cell forward. (d) Filopodia: thin membrane extension made of parallel bundles, and pro-
jected forward to sense the mechanical properties of the substrate. Each of these structures has its proper mechanical behavior: (A) Contractile
elements activated by the myosin motor. (B) Viscoelastic elements based on a Kelvin-Voigt model (viscous damper and elastic spring connected
in parallel). (C) Stiff elements associated with filopodium. Here the cell is represented on a 2D substrate instead of the 3D real ECM. That is
why the cell has adopted an apico-basal polarity. The apical side refers to the unattached membrane above the nucleus, and the basal one to the
contact with the substrate, through focal adhesions. Based on the lecture of E. Planus, Cours de M1, 2017 (non-disponible en acc€ as libre).
I. BIOMECHANICS