Page 107 - Advances in Biomechanics and Tissue Regeneration
P. 107
6.3 ACTIVE BIOMECHANICAL BEHAVIOR 103
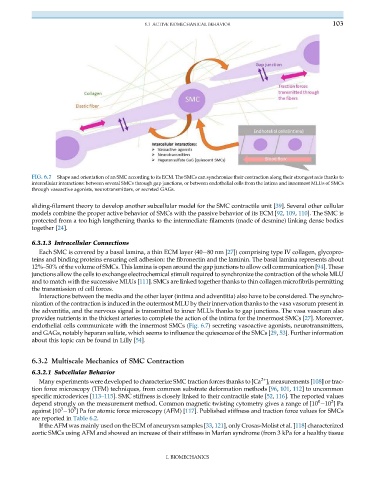
FIG. 6.7 Shape and orientation of an SMC according to its ECM. The SMCs can synchronize their contraction along their strongest axis thanks to
intercellular interactions: between several SMCs through gap junctions, or between endothelial cells from the intima and innermost MLUs of SMCs
through vasoactive agonists, neurotransmitters, or secreted GAGs.
sliding-filament theory to develop another subcellular model for the SMC contractile unit [39]. Several other cellular
models combine the proper active behavior of SMCs with the passive behavior of its ECM [92, 109, 110]. The SMC is
protected from a too high lengthening thanks to the intermediate filaments (made of desmine) linking dense bodies
together [24].
6.3.1.3 Intracellular Connections
Each SMC is covered by a basal lamina, a thin ECM layer (40 80 nm [27]) comprising type IV collagen, glycopro-
teins and binding proteins ensuring cell adhesion: the fibronectin and the laminin. The basal lamina represents about
12%–50% of the volume of SMCs. This lamina is open around the gap junctions to allow cell communication [94]. These
junctions allow the cells to exchange electrochemical stimuli required to synchronize the contraction of the whole MLU
and to match with the successive MLUs [111]. SMCs are linked together thanks to thin collagen microfibrils permitting
the transmission of cell forces.
Interactions between the media and the other layer (intima and adventitia) also have to be considered. The synchro-
nization of the contraction is induced in the outermost MLU by their innervation thanks to the vasa vasorum present in
the adventitia, and the nervous signal is transmitted to inner MLUs thanks to gap junctions. The vasa vasorum also
provides nutrients in the thickest arteries to complete the action of the intima for the innermost SMCs [27]. Moreover,
endothelial cells communicate with the innermost SMCs (Fig. 6.7) secreting vasoactive agonists, neurotransmitters,
and GAGs, notably heparan sulfate, which seems to influence the quiescence of the SMCs [29, 53]. Further information
about this topic can be found in Lilly [54].
6.3.2 Multiscale Mechanics of SMC Contraction
6.3.2.1 Subcellular Behavior
2+
Many experiments were developed to characterize SMC traction forces thanks to [Ca ] i measurements [108] or trac-
tion force microscopy (TFM) techniques, from common substrate deformation methods [96, 101, 112] to uncommon
specific microdevices [113–115]. SMC stiffness is closely linked to their contractile state [52, 116]. The reported values
2
0
depend strongly on the measurement method. Common magnetic twisting cytometry gives a range of [10 10 ]Pa
5
3
against [10 10 ] Pa for atomic force microscopy (AFM) [117]. Published stiffness and traction force values for SMCs
are reported in Table 6.2.
If the AFM was mainly used on the ECM of aneurysm samples [33, 121], only Crosas-Molist et al. [118] characterized
aortic SMCs using AFM and showed an increase of their stiffness in Marfan syndrome (from 3 kPa for a healthy tissue
I. BIOMECHANICS