Page 357 - Advances in Biomechanics and Tissue Regeneration
P. 357
354 17. SKIN MECHANOBIOLOGY AND BIOMECHANICS: FROM HOMEOSTASIS TO WOUND HEALING
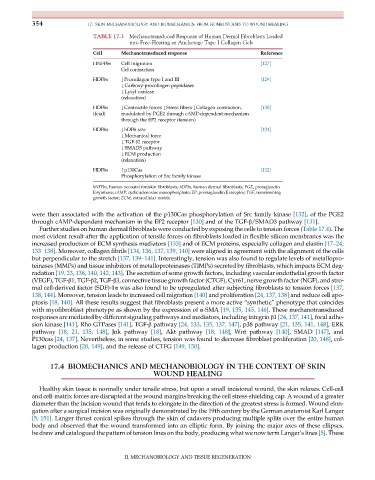
TABLE 17.3 Mechanotransduced Response of Human Dermal Fibroblasts Loaded
into Free-Floating or Anchorage Type I Collagen Gels
Cell Mechanotransduced response Reference
HNFFbs Cell migration [127]
Gel contraction
HDFbs #Procollagen type I and III [129]
#Carboxy-procollagen peptidases
#Lysyl oxidase
(relaxation)
HDFbs #Contractile forces #Stress fibers #Collagen contraction, [130]
(fetal) modulated by PGE2 through cAMP-dependent mechanism
through the EP2 receptor (tension)
HDFbs #hDFb size [131]
#Mechanical force
#TGF-β2 receptor
#SMAD3 pathway
#ECM production
(relaxation)
HDFbs "p130Cas [132]
Phosphorylation of Src family kinase
hNFFbs, human neonatal foreskin fibroblasts; hDFbs, human dermal fibroblasts; PGE, protaglandin
E synthase; cAMP, cyclic adenosine monophosphate; EP, prostaglandin E receptor; TGF, transforming
growth factor; ECM, extracellular matrix.
were then associated with the activation of the p130Cas phosphorylation of Src family kinase [132], of the PGE2
through cAMP-dependent mechanism in the EP2 receptor [130] and of the TGF-β/SMAD3 pathway [131].
Further studies on human dermal fibroblasts were conducted by exposing the cells to tension forces (Table 17.4). The
most evident result after the application of tensile forces on fibroblasts loaded in flexible silicon membranes was the
increased production of ECM synthesis mediators [133] and of ECM proteins, especially collagen and elastin [17–24,
133–138]. Moreover, collagen fibrils [134, 136, 137, 139, 140] were aligned in agreement with the alignment of the cells
but perpendicular to the stretch [137, 139–141]. Interestingly, tension was also found to regulate levels of metallopro-
teinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) secreted by fibroblasts, which impacts ECM deg-
radation [19, 23, 138, 140, 142, 143]. The secretion of some growth factors, including vascular endothelial growth factor
(VEGF), TGF-β1, TGF-β2, TGF-β3, connective tissue growth factor (CTGF), Cyr61, nerve growth factor (NGF), and stro-
mal cell-derived factor (SDF)-1α was also found to be upregulated after subjecting fibroblasts to tension forces [137,
138, 144]. Moreover, tension leads to increased cell migration [140] and proliferation [24, 137, 138] and reduce cell apo-
ptosis [18, 140]. All these results suggest that fibroblasts present a more active “synthetic” phenotype that coincides
with myofibroblast phenotype as shown by the expression of α-SMA [19, 135, 145, 146]. These mechanotransduced
responses are mediated by different signaling pathways and mediators, including integrin β1 [24, 137, 141], focal adhe-
sion kinase [141], Rho GTPases [141], TGF-β pathway [24, 133, 135, 137, 147], p38 pathway [21, 135, 141, 148], ERK
pathway [18, 21, 135, 148], Jnk pathway [18], Akt pathway [18, 148], Wnt pathway [140], SMAD [147], and
P130cas [24, 137]. Nevertheless, in some studies, tension was found to decrease fibroblast proliferation [20, 148], col-
lagen production [20, 149], and the release of CTFG [149, 150].
17.4 BIOMECHANICS AND MECHANOBIOLOGY IN THE CONTEXT OF SKIN
WOUND HEALING
Healthy skin tissue is normally under tensile stress, but upon a small incisional wound, the skin relaxes. Cell-cell
and cell-matrix forces are disrupted at the wound margins breaking the cell stress-shielding cap. A wound of a greater
diameter than the incision wound that tends to elongate in the direction of the greatest stress is formed. Wound elon-
gation after a surgical incision was originally demonstrated by the 19th century by the German anatomist Karl Langer
[5, 151]. Langer thrust conical spikes through the skin of cadavers producing multiple splits over the entire human
body and observed that the wound transformed into an elliptic form. By joining the major axes of these ellipses,
he drew and catalogued the pattern of tension lines on the body, producing what we now term Langer’s lines [5]. These
II. MECHANOBIOLOGY AND TISSUE REGENERATION