Page 66 - An Introduction to Microelectromechanical Systems Engineering
P. 66
Basic Process Tools 45
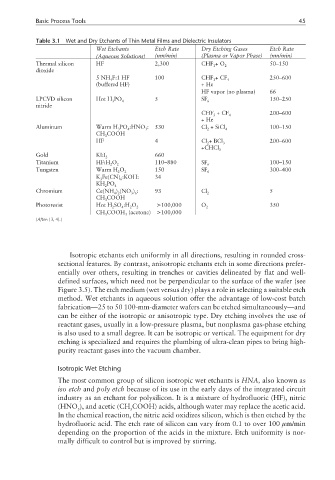
Table 3.1 Wet and Dry Etchants of Thin Metal Films and Dielectric Insulators
Wet Etchants Etch Rate Dry Etching Gases Etch Rate
(Aqueous Solutions) (nm/min) (Plasma or Vapor Phase) (nm/min)
Thermal silicon HF 2,300 CHF +O 2 50–150
3
dioxide
5NH F:1 HF 100 CHF +CF 4 250–600
3
4
(buffered HF) +He
HF vapor (no plasma) 66
LPCVD silicon Hot H PO 4 5 SF 6 150–250
3
nitride
CHF +CF 4 200–600
3
+He
Aluminum Warm H PO :HNO : 530 Cl + SiCl 4 100–150
4
3
2
3
CH COOH
3
HF 4 Cl + BCl 3 200–600
2
+CHCl 3
Gold KI:I 2 660
Titanium HF:H O 2 110–880 SF 6 100–150
2
Tungsten Warm H O 2 150 SF 6 300–400
2
K Fe(CN) :KOH: 34
3
6
KH PO 4
2
Chromium Ce(NH ) (NO ) : 93 Cl 2 5
4 2
3 6
CH COOH
3
Photoresist Hot H SO :H O 2 >100,000 O 2 350
4
2
2
CH COOH (acetone) >100,000
3
3
(After: [3, 4].)
Isotropic etchants etch uniformly in all directions, resulting in rounded cross-
sectional features. By contrast, anisotropic etchants etch in some directions prefer-
entially over others, resulting in trenches or cavities delineated by flat and well-
defined surfaces, which need not be perpendicular to the surface of the wafer (see
Figure 3.5). The etch medium (wet versus dry) plays a role in selecting a suitable etch
method. Wet etchants in aqueous solution offer the advantage of low-cost batch
fabrication—25 to 50 100-mm-diameter wafers can be etched simultaneously—and
can be either of the isotropic or anisotropic type. Dry etching involves the use of
reactant gases, usually in a low-pressure plasma, but nonplasma gas-phase etching
is also used to a small degree. It can be isotropic or vertical. The equipment for dry
etching is specialized and requires the plumbing of ultra-clean pipes to bring high-
purity reactant gases into the vacuum chamber.
Isotropic Wet Etching
The most common group of silicon isotropic wet etchants is HNA, also known as
iso etch and poly etch because of its use in the early days of the integrated circuit
industry as an etchant for polysilicon. It is a mixture of hydrofluoric (HF), nitric
(HNO ), and acetic (CH COOH) acids, although water may replace the acetic acid.
3 3
In the chemical reaction, the nitric acid oxidizes silicon, which is then etched by the
hydrofluoric acid. The etch rate of silicon can vary from 0.1 to over 100 µm/min
depending on the proportion of the acids in the mixture. Etch uniformity is nor-
mally difficult to control but is improved by stirring.