Page 243 - Battery Reference Book
P. 243
Nickel-cadmium secondary batteries 19/9
I Inches Millimetres Nore :
POSITION Of TABS IN PGLATIoN TO
ONE AN0THe.P TO ,915 WITHIN r5°
0024 -
1.63
0 069 1.75
0 174
0 119 3.02
3.18
4.76
0213
0 >>3 5.66
0378 -
0 457 11 6
--+ ,378"
+.ooo"
- . 00 8 "
0"
-.o 0 e/'
Flat contacts Two solder tabs
(-) (-1
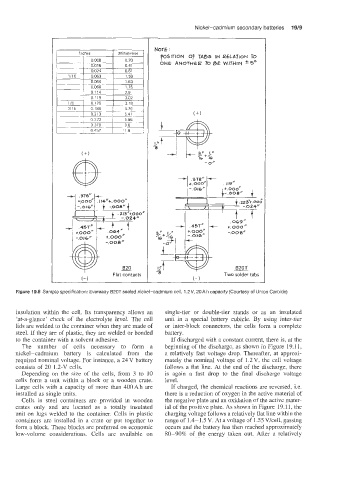
Figure 19.8 Sample specification: Eveready B20T sealed nickel-cadmium cell, 1.2V, 20A h capacity (Courtesy of Union Carbide)
insulation within the cell. Its transparency allows an single-tier or double-tier stands or as an insulated
'at-a-glance' check of the electrolyte level. The cell unit in a special battery cubicle. By using inter-tier
lids are welded to the container when they are made of or inter-block connectors, the cells form a complete
steel. If they are of plastic, they are welded or bonded battery.
to the container with a solvent adhesive. If discharged with a constant current, there is, at the
The number of cells necessary to form a beginning of the discharge, as shown in Figure 19.11,
nickel-cadmium battery is calculated from the a relatively fast voltage drop. Thereafter, at approxi-
required nominal voltage. For instance, a 24 V battery mately the nominal voltage of 1.2 V, the cell voltage
consists of 20 1.2-V cells. follows a flat line. At the end of the discharge, there
Depending on the size of the ceIls, from 3 to 10 is again a fast drop to the final discharge voltage
cells form a unit within a block or a wooden crate. level.
Large cells with a capacity of more than 400 Ah are If charged, the chemical reactions are reversed, i.e.
installed as single units. there is a reduction of oxygen in the active material of
Cells in steel containers are provided in wooden the negative plate and an oxidation of the active mater-
crates only and are located as a totally insulated ial of the positive plate. As shown in Figure 19.11, the
unit on lugs welded to the container. Cells in plastic charging voltage follows a relatively flat line within the
containers are installed in a crate or put together to range of 1.4-1.5 V. At a voltage of 1.55 V/cell, gassing
form a block. These blocks are preferred on economic occurs and the battery has then reached approximately
low-volume considerations. Cells are available on 80-90% of the energy taken out. After a relatively