Page 245 - Battery Reference Book
P. 245
Nickel-cadmium secondary batteries 19/11
1.8 The alkaline electrolyte in nickel-cadmium batter-
1.7 ies keeps its density and low freezing point constant
1.6 when discharging. As a lead-acid battery runs down,
- 1.5 the density of the sulphuric acid decreases, which
-
2 1.4 means that the electrolyte freezes at low tempera-
3 tures and the battery is damaged. Nickel-cadmium
I 1.3
batteries therefore perform better at low tempera-
3 1.2 tures than lead-acid batteries. The risk of damage to
1.1
0.9 i \ the batteries from cold is therefore practically non-
existent.
The life of nickel-cadmium batteries is also less
-
l.o
affected by high temperatures than that of lead-acid
batteries. These batteries work in both tropical heat
and arctic cold. The battery range covers temperatures
from -50°C to +55"@.
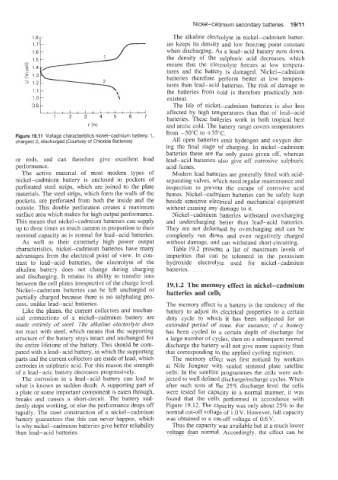
Figure 10.1 1 Voltage characteristics nickel-cadmium battery: 1,
charged; 2, disch,arged (Courtesy of Chloride Batteries) All open batteries emit hydrogen and oxygen dur-
ing the final stage of charging. In nickel-cadmium
batteries these are the only gases given off, whereas
or rods, and can therefore give excellent load lead-acid batteries also give off corrosive sulphuric
performance. acid fumes.
The active material of most modem types of Modern lead batteries are generally fitted with acid-
nickel-cadmium battery is enclosed in pockets of separating valves, which need regular maintenance and
perforated steel strips, which are joined to the plate inspection to prevent the escape of corrosive acid
materials. The steel strips, which form the walls of the fumes. Nickel-cadmium batteries can be safely kept
pockets, are perforated from both the inside and the beside sensitive electrical and mechanical equipment
outside. This double perforation creates a maximum without causing any damage to it.
surface area which makes for high output performance. Nickel-cadmium batteries withstand overcharging
This means that nickel-cadmium batteries can supply and undercharging better than lead-acid batteries.
up to three times as much current in proportion to their They are not deformed by overcharging and can be
nominal capacity as is normal for lead-acid batteries. completely run down and even negatively charged
As well as their extremely high power output without damage, and can withstand short-circuiting.
characteristics, nickel-cadmium batteries have many Table 19.2 presents a list of maximum levels of
advantages from the electrical point of view. In con- impurities that can be tolerated in the potassium
trast to lead-acid batteries, the electrolyte of the hydroxide electrolyte used for nickel-cadmium
alkaline battery does not change during charging batteries.
and discharging. It retains its ability to transfer ions
between the cell plates irrespective of the charge level. 19.1.2 The memory effect in nickel-cadmium
Nickel-cadmium batteries can be left uncharged or batteries and cells
partially charged because there is no sulphating pro-
cess, unlike lead-acid batteries. The memory effect in a battery is the tendency of the
Like the plates, the current collectors and mechm- battery to adjust its electrical properties to a certain
icd connections of a nickel-cadmium battery are duty cycle to which it has been subjected for an
made entirely of steel. The alkaline electrolyte does extended period of time. For instance, if a battery
not react with steel, which means that the supporting has been cycled to a certain depth of discharge for
structure of the battery stays intact and unchanged for a large number of cycles, then on a subsequent normal
the entire lifetime of the battery. This should be com- discharge the battery will not give more capacity than
pared with a lead-acid battery, in which the supporting that corresponding to the applied cycling regimen.
parts and the current collectors are made of lead, which The memory effect was first noticed by workers
corrodes in suliphu~ic acid. For this reason the strength at Nife Jungner with sealed sintered plate satellite
of a lead-acid. battery decreases progressively. cells. In the satellite programmes the cells were sub-
The corrosion in a lead-acid battery can lead to jected to well defined dischargehecharge cycles. When
what is known as sudden death. A supporting part of after such tests at the 25% discharge level the cells
a plate or some important component is eaten through, were tested for capacity in a normal manner, it was
breaks and causes a short-circuit. The battery sud- found that the cells performed in accordance with
denly stops working, or else the performance drops off Figure 19.12. The capacity was only about 25% to the
rapidly. The steel construction of a nickel-cadmium normal cut-off voltage of 1 .O V. However, full capacity
battery guarantees that this can never happen, which was obtained to a cut-off voltage of 0.6 V.
is why nickelkadmiurn batteries give better reliability Thus the capacity was available but at a much lower
than lead-acid batteries. voltage than normal. Accordingly, the effect can be