Page 246 - Battery Reference Book
P. 246
19/12 Nickel batteries
Table 19.2 Maximum allowable impurity levels in potassium sealed and vented sintered plate cells. Pocket plate
hydroxide electrolyte (relative density 1.28) used in nickel-cadmium cells and batteries do not develop the
nickel-cadmium batteries memory effect under any circumstances.
Although it is not fully clear what causes the mem-
Impurities Moximum ory effect, indications are that memory is connected
concentration mainly with physical changes in the negative electrode
(mg4
involving the formation of intermetallic compounds of
1. Metals of the hydrochloric acid and nickel and cadmium. These are produced by interac-
hydrogen sulphide group, e.g. tions between charged, negative active material and
arsenic, antimony, tin, bismuth, the large surface area of the sintered nickel support.
cadmium, mercury, silver, copper, Scanning electron micrographs have shown that nega-
lead 5 tive electrodes with memory contain a greater number
2. Metals of the ammonium sulphide of large cadmium hydroxide particles than do nor-
group, e.g. iron, aluminium, mal negative electrodes. During discharge the reactions
chromium, manganese, zinc. cobalt, proceed uniformly when the cadmium active material
nickel 20
3. Sodium 5000 consists of very small particles as is the case in a cell
4. Halogen and cyanide 150 without memory effect. On the other hand, if the cad-
5. Nitrogen 100 mium material contains both smaller and larger crys-
6. Sulphur 5 tals, as in a cell with memory, the smaller particles will
7. Carbon dioxide 1000 react first and become discharged at a normal voltage,
8. Oxidizable carbon (in organic and then the larger particles will discharge. Because the
compounds) 50 current density will be higher during the discharge of
9. Silicic acid (SiOz) 50 the larger particles, the polarization will increase and
10. Other non-metals, e.g. phosphorus, there will be a loss of voltage in the cell. Accordingly,
boron 10 the discharge curve will exhibit two voltage plateaux.
The reasons why the cadmium active material
changes its particle size distribution during memory
cycling are not fully understood. It is well known
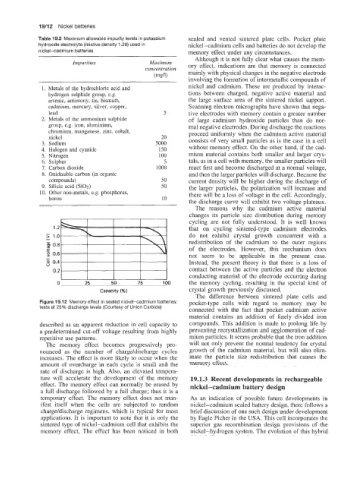
- 1.2 that on cycling sintered-type cadmium electrodes
> 1.0 do not exhibit crystal growth concurrent with a
.....
0 0.8 redistribution of the cadmium to the outer regions
4- - of the electrodes. However, this mechanism does
0.6 not seem to be applicable in the present case.
-
5 0.4 Instead, the present theory is that there is a loss of
u
0.2 contact between the active particles and the electron
conducting material of the electrode occurring during
0 25 50 75 the memory cycling, resulting in the special kind of
Capacity (%I crystal growth previously discussed.
The difference between sintered plate cells and
Figure 19.12 Memory effect in sealed nickel-cadmium batteries: pocket-type cells with regard to memory may be
tests at 25% discharge levels (Courtesy of Union Carbide) connected with the fact that pocket cadmium active
material contains an addition of finely divided iron
described as an apparent reduction in cell capacity to compounds. This addition is made to prolong life by
a predetermined cut-off voltage resulting from highly preventing recrystallization and agglomeration of cad-
repetitive use patterns. mium particles. It seems probable that the iron addition
The memory effect becomes progressively pro- will not only prevent the normal tendency for crystal
nounced as the number of charge/discharge cycles growth of the cadmium material, but will also elim-
increases. The effect is more likely to occur when the inate the particle size redistribution that causes the
amount of overcharge in each cycle is small and the memory effect.
rate of discharge is high. Also, an elevated tempera-
ture will accelerate the development of the memory 19.1.3 Recent developments in rechargeable
effect. The memory effect can normally be erased by nickel-cadmium battery design
a full discharge followed by a full charge; thus it is a
temporary effect. The memory effect does not man- As an indication of possible future developments in
ifest itself when the cells are subjected to random nickel-cadmium sealed battery design, there follows a
charge/discharge regimens, which is typical for most brief discussion of one such design under development
applications. It is important to note that it is only the by Eagle Picher in the USA. This cell incorporates the
sintered type of nickel-cadmium cell that exhibits the superior gas recombination design provisions of the
memory effect. The effect has been noticed in both nickel-hydrogen system. The evolution of this hybrid