Page 273 - Fluid mechanics, heat transfer, and mass transfer
P. 273
CONVECTIVE HEAT TRANSFER BASICS
254
& Although film boiling is not desirable because of low
9.1.3 Boiling and Vaporization
heat transfer coefficients involved, it is unavoidable
. Explain the phenomena of (i) nucleate boiling and (ii) in some situations as in the vaporization of low
film boiling and discuss the effects of these two boiling boiling liquids or in cryogenic vaporizers.
phenomena on heat transfer rates. & Film boiling might appear attractivefrom fouling and
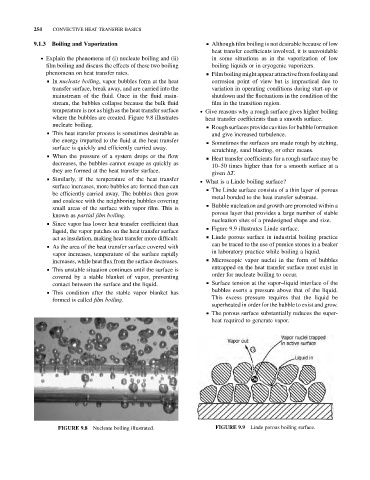
& In nucleate boiling, vapor bubbles form at the heat corrosion point of view but is impractical due to
transfer surface, break away, and are carried into the variation in operating conditions during start-up or
mainstream of the fluid. Once in the fluid main- shutdown and the fluctuations in the condition of the
stream, the bubbles collapse because the bulk fluid film in the transition region.
temperature is not as high as the heat transfer surface . Give reasons why a rough surface gives higher boiling
where the bubbles are created. Figure 9.8 illustrates heat transfer coefficients than a smooth surface.
nucleate boiling. & Rough surfaces provide cavities for bubble formation
& This heat transfer process is sometimes desirable as
and give increased turbulence.
the energy imparted to the fluid at the heat transfer & Sometimes the surfaces are made rough by etching,
surface is quickly and efficiently carried away.
scratching, sand blasting, or other means.
& When the pressure of a system drops or the flow
& Heat transfer coefficients for a rough surface may be
decreases, the bubbles cannot escape as quickly as
10–50 times higher than for a smooth surface at a
they are formed at the heat transfer surface.
given DT.
& Similarly, if the temperature of the heat transfer
. What is a Linde boiling surface?
surface increases, more bubbles are formed than can
& The Linde surface consists of a thin layer of porous
be efficiently carried away. The bubbles then grow
metal bonded to the heat transfer substrate.
and coalesce with the neighboring bubbles covering
& Bubble nucleation and growth are promoted within a
small areas of the surface with vapor film. This is
known as partial film boiling. porous layer that provides a large number of stable
nucleation sites of a predesigned shape and size.
& Since vapor has lower heat transfer coefficient than
& Figure 9.9 illustrates Linde surface.
liquid, the vapor patches on the heat transfer surface
act as insulation, making heat transfer more difficult. & Linde porous surface in industrial boiling practice
& As the area of the heat transfer surface covered with can be traced to the use of pumice stones in a beaker
in laboratory practice while boiling a liquid.
vapor increases, temperature of the surface rapidly
increases, while heat flux from the surface decreases. & Microscopic vapor nuclei in the form of bubbles
entrapped on the heat transfer surface must exist in
& This unstable situation continues until the surface is
order for nucleate boiling to occur.
covered by a stable blanket of vapor, preventing
contact between the surface and the liquid. & Surface tension at the vapor–liquid interface of the
bubbles exerts a pressure above that of the liquid.
& This condition after the stable vapor blanket has
This excess pressure requires that the liquid be
formed is called film boiling.
superheated in order for the bubble to exist and grow.
& The porous surface substantially reduces the super-
heat required to generate vapor.
Nucleate boiling illustrated. Linde porous boiling surface.
FIGURE 9.8 FIGURE 9.9