Page 600 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 600
Membrane Processes 555
Example 17.1 Calculation of Osmotic Pressure Calculation by the spreadsheet, Table CD17.6, gives
p ¼ 0.42 atm (43 kPa or 6 psi). In other words, the mem-
Given brane flux is proportional to the transmembrane minus p.
A solution of MgCl 2 has a concentration of 1000 mg=L. Let
the temperature of the solution be 258C. 17.3.6.3 Effect of Membrane Pressure on Water
Required Flux Density
Calculate the osmotic pressure.
RO forces a solvent from the solution side, as depicted in Figure
Solution 17.15c, to the pure solvent side by application of a pressure
A spreadsheet, Table CD17.6, shows the sequence of calcu- higher than the osmotic pressure. The flux is proportional to the
lations. Starting with the compound, the molecular weight is pressure difference between the two sides of the membrane
determined (based on data in Table B.1). The molar concen- minus the osmotic pressure, that is, the net driving pressure
tration of the compound itself is given. But when dissolved, (DP p), to give,
the compound dissociates into ions, giving three ions per
molecule. Therefore, the multiplier is ‘‘3’’ to give a total K(membrane)
molar concentration given in the next column. Next, the (DP p) (17:16)
j ¼
van’t Hoff equation, Equation 17.15, is applied to give the m
w
osmotic pressure, p, in kPa (using R ¼ 8314.510 Pa-L=
g-mol K). The van’t Hoff equation must be divided by 1000 where
since the units are kPa (i.e., not Pa). Divide by 101.325 DP is the pressure difference between the two sides of
kPa=atm to obtain the osmotic pressure in atmospheres. membrane (kPa)
Discussion p is the osmotic pressure of solute side of membrane (kPa)
The spreadsheet shows the van’t Hoff calculation for
several compounds and may be used for other calcula- The equation applies to any membrane that is not perme-
tions that may be of interest. Consider, for example, water able to salt. Technically, the term p should be Dp,where
with TDS 500 mg=L. Let NaCl serve as a surrogate. Dp ¼ p(salt side) p(pure water side). But since, as a rule,
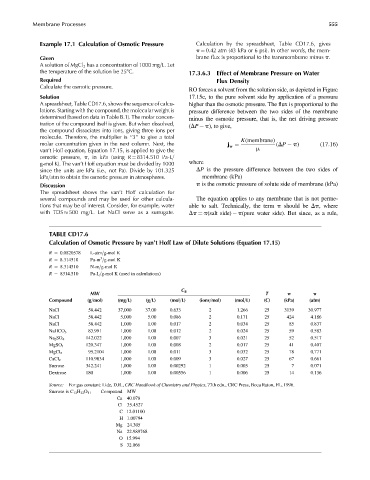
TABLE CD17.6
Calculation of Osmotic Pressure by van’t Hoff Law of Dilute Solutions (Equation 17.15)
R ¼ 0.0820578 L-atm=g-mol K
3
R ¼ 8.314510 Pa-m =g-mol K
R ¼ 8.314510 N-m=g-mol K
R ¼ 8314.510 Pa-L=g-mol K (used in calculations)
C B
MW T p p
Compound (g=mol) (mg=L) (g=L) (mol=L) (ions=mol) (mol=L) (C) (kPa) (atm)
NaCl 58.442 37,000 37.00 0.633 2 1.266 25 3139 30.977
NaCl 58.442 5,000 5.00 0.086 2 0.171 25 424 4.186
NaCl 58.442 1,000 1.00 0.017 2 0.034 25 85 0.837
83.991 1,000 1.00 0.012 2 0.024 25 59 0.583
NaHCO 3
Na 2 SO 4 142.022 1,000 1.00 0.007 3 0.021 25 52 0.517
120.347 1,000 1.00 0.008 2 0.017 25 41 0.407
MgSO 4
MgCl 2 95.2104 1,000 1.00 0.011 3 0.032 25 78 0.771
110.9834 1,000 1.00 0.009 3 0.027 25 67 0.661
CaCl 2
Sucrose 342.241 1,000 1.00 0.00292 1 0.003 25 7 0.071
Dextrose 180 1,000 1.00 0.00556 1 0.006 25 14 0.136
Source: For gas constant: Lide, D.R., CRC Handbook of Chemistry and Physics, 77th edn., CRC Press, Boca Raton, FL, 1996.
Sucrose is C 12 H 22 O 11 Compound MW
Ca 40.078
Cl 35.4527
C 12.01100
H 1.00794
Mg 24.305
Na 22.989768
O 15.994
S 32.066