Page 602 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 602
Membrane Processes 557
17.3.8.1 Reversible and Irreversible Fouling 17.3.8.3 Particle Fouling
Membranes invariably foul with time. Figure 17.18 illustrates Particles may deposit on membrane surfaces or collect within
four fouling cycles, in which flux declines with time but is membrane pores. Deposited particles may remain near the
restored partially by cleaning. The fouling cycle may be surface (e.g., gel-polarization layer or pore blockage) or they
defined by three fouling terms, that is, (1) ‘‘total’’ fouling, may be transported back into the bulk flow by diffusion after
seen as the overall loss of flux; (2) reversible fouling, which the removal by shear. At steady state, the net transport of
is that part of total fouling that may be restored by cleaning; and particles toward the membrane is in balance with the back-
(3) irreversible fouling, which is that part of total fouling that is transport mechanisms (i.e., Brownian diffusion and turbulent
not restored by cleaning. For spiral-wound membranes, the diffusion if the advective flow is in the turbulent regime).
time between the cleaning cycles depends on the feed-water Calculations performed by Wiesner and Chellam (1992)
quality, which depends, in turn, on the ambient water quality describing flow through thin plates showed that particles
and the pretreatment. For spiral-wound membranes, the time near 0.1 mm in radius would preferentially accumulate on
between cleaning events may vary from weeks to months. Over the surface of the membrane. Theoretically, particles less
time, however, irreversible fouling increases to such extent that than 0.1 mm in radius are transported primarily by Brownian
replacement becomes economical. In the case of the RO mem- diffusion, while particles greater than 0.1 mm in radius are
branes at Brighton, Colorado, the membranes were replaced transported primarily by turbulent diffusion.
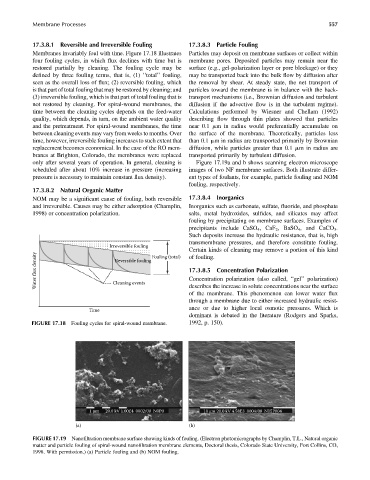
only after several years of operation. In general, cleaning is Figure 17.19a and b shows scanning electron microscope
scheduled after about 10% increase in pressure (increasing images of two NF membrane surfaces. Both illustrate differ-
pressure is necessary to maintain constant flux density). ent types of foulants, for example, particle fouling and NOM
fouling, respectively.
17.3.8.2 Natural Organic Matter
NOM may be a significant cause of fouling, both reversible 17.3.8.4 Inorganics
and irreversible. Causes may be either adsorption (Champlin, Inorganics such as carbonate, sulfate, fluoride, and phosphate
1998) or concentration polarization. salts, metal hydroxides, sulfides, and silicates may affect
fouling by precipitating on membrane surfaces. Examples of
precipitants include CaSO 4 , CaF 2 , BaSO 4 , and CaCO 3 .
Such deposits increase the hydraulic resistance, that is, high
transmembrane pressures, and therefore constitute fouling.
Irreversible fouling
Certain kinds of cleaning may remove a portion of this kind
Water flux density Reversible fouling 17.3.8.5 Concentration Polarization
Fouling (total)
of fouling.
Concentration polarization (also called, ‘‘gel’’ polarization)
Cleaning events
describes the increase in solute concentrations near the surface
of the membrane. This phenomenon can lower water flux
through a membrane due to either increased hydraulic resist-
ance or due to higher local osmotic pressures. Which is
Time
dominant is debated in the literature (Rodgers and Sparks,
FIGURE 17.18 Fouling cycles for spiral-wound membrane. 1992, p. 150).
1 μm 20.0 kV 1.00E4 0002/00 N0P0 10 μm 20.0 kV 4.58E3 0004/00 N15P004
(a) (b)
FIGURE 17.19 Nanofiltration membrane surface showing kinds of fouling. (Electron photomicrographs by Champlin, T.L., Natural organic
matter and particle fouling of spiral-wound nanofiltration membrane elements, Doctoral thesis, Colorado State University, Fort Collins, CO,
1998. With permission.) (a) Particle fouling and (b) NOM fouling.