Page 281 - Geothermal Energy Systems Exploration, Development, and Utilization
P. 281
5.3 Reservoir Characterization 257
Supercritical
CO 2
Liquid P C 7.28 MPa
Solid
Pressure
Vapour
Temperature 304.2 K
T 31.2 °C
C
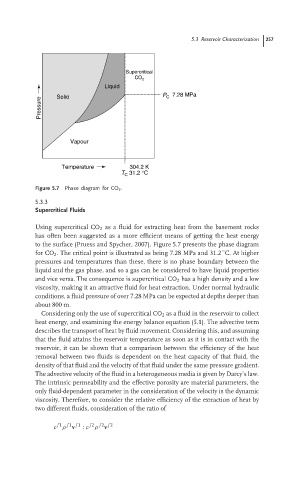
Figure 5.7 Phase diagram for CO 2 .
5.3.3
Supercritical Fluids
Using supercritical CO 2 as a fluid for extracting heat from the basement rocks
has often been suggested as a more efficient means of getting the heat energy
to the surface (Pruess and Spycher, 2007). Figure 5.7 presents the phase diagram
◦
for CO 2 . The critical point is illustrated as being 7.28 MPa and 31.2 C. At higher
pressures and temperatures than these, there is no phase boundary between the
liquid and the gas phase, and so a gas can be considered to have liquid properties
and vice versa. The consequence is supercritical CO 2 has a high density and a low
viscosity, making it an attractive fluid for heat extraction. Under normal hydraulic
conditions, a fluid pressure of over 7.28 MPa can be expected at depths deeper than
about 800 m.
Considering only the use of supercritical CO 2 as a fluid in the reservoir to collect
heat energy, and examining the energy balance equation (5.1). The advective term
describes the transport of heat by fluid movement. Considering this, and assuming
that the fluid attains the reservoir temperature as soon as it is in contact with the
reservoir, it can be shown that a comparison between the efficiency of the heat
removal between two fluids is dependent on the heat capacity of that fluid, the
density of that fluid and the velocity of that fluid under the same pressure gradient.
The advective velocity of the fluid in a heterogeneous media is given by Darcy’s law.
The intrinsic permeability and the effective porosity are material parameters, the
only fluid-dependent parameter in the consideration of the velocity is the dynamic
viscosity. Therefore, to consider the relative efficiency of the extraction of heat by
two different fluids, consideration of the ratio of
f 2 f 2
f 2
f 1
f 1 f 1
c ρ v : c ρ v