Page 31 - Handbook of Surface Improvement and Modification
P. 31
26 Scratch and Mar Resistance
2.2.11 SILSESQUIOXANE
GENERAL INFORMATION
Name: silsesquioxane CAS #: 5177-38-9 Active ingredient, wt%: 97
Molecular size, nm: 1-5 Molecular mass: 791-1322
PHYSICAL PROPERTIES
State: solid, wax, liquid Odor: odorless Color: colorless
o
o
Thermal stability, C: 250-350 (up to > 400) T , C: 98-102
g
3
Density, kg/m : 0.9-1.3 (up to 1.82) Particle size, nm: 1.5-100
2
Specific surface area, m /g: 3600 Refractive index: 1.4-165
USE & PERFORMANCE
Recommended for polymers: acrylics, epoxy, melamine, PMMA, PU
Recommended for products: clearcoats
Concentrations used, wt%: 1-3
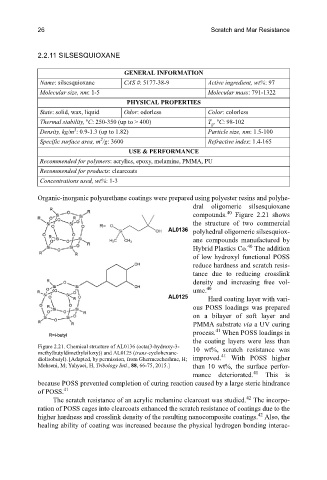
Organic-inorganic polyurethane coatings were prepared using polyester resins and polyhe-
dral oligomeric silsesquioxane
40
compounds. Figure 2.21 shows
the structure of two commercial
polyhedral oligomeric silsesquiox-
ane compounds manufactured by
40
Hybrid Plastics Co. The addition
of low hydroxyl functional POSS
reduce hardness and scratch resis-
tance due to reducing crosslink
density and increasing free vol-
40
ume.
Hard coating layer with vari-
ous POSS loadings was prepared
on a bilayer of soft layer and
PMMA substrate via a UV curing
41
process. When POSS loadings in
the coating layers were less than
Figure 2.21. Chemical structure of AL0136 (octa(3-hydroxy-3- 10 wt%, scratch resistance was
methylbutyldimethylsiloxy)) and AL0125 (trans-cyclohexane- 41
diolisobutyl). [Adapted, by permission, from Ghermezcheshme, H; improved. With POSS higher
Mohseni, M; Yahyaei, H, Tribology Intl., 88, 66-75, 2015.] than 10 wt%, the surface perfor-
41
mance deteriorated. This is
because POSS prevented completion of curing reaction caused by a large steric hindrance
41
of POSS.
42
The scratch resistance of an acrylic melamine clearcoat was studied. The incorpo-
ration of POSS cages into clearcoats enhanced the scratch resistance of coatings due to the
42
higher hardness and crosslink density of the resulting nanocomposite coatings. Also, the
healing ability of coating was increased because the physical hydrogen bonding interac-