Page 273 - Hydrogeology Principles and Practice
P. 273
HYDC07 12/5/05 5:32 PM Page 256
256 Chapter Seven
gases, and long-term studies are required for the full physical, chemical or biological processes that, under
evaluation of PRBs (Box 7.3). favourable conditions, act without human interven-
tion to reduce the mass, toxicity, mobility, volume or
concentration of contaminants in soil or groundwater.
7.2.3 Monitored natural attenuation These in situ processes include biodegradation, dis-
persion, dilution, sorption, volatilization, radioactive
The United States Environmental Protection Agency decay, and chemical or biological stabilization, trans-
(1997) defined natural attenuation as a variety of formation or destruction of contaminants (Fig. 7.2).
BO X
In situ permeable reactive barrier for remediation of chlorinated solvents
7. 3
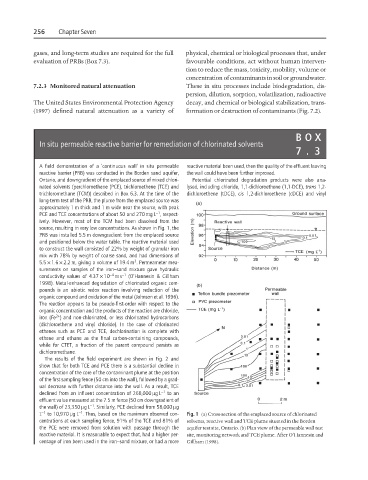
A field demonstration of a ‘continuous wall’ in situ permeable reactive material been used, then the quality of the effluent leaving
reactive barrier (PRB) was conducted in the Borden sand aquifer, the wall could have been further improved.
Ontario, and downgradient of the emplaced source of mixed chlori- Potential chlorinated degradation products were also ana-
nated solvents (perchloroethene (PCE), trichloroethene (TCE) and lysed, including chloride, 1,1-dichloroethene (1,1-DCE), trans 1,2-
trichloromethane (TCM)) described in Box 6.3. At the time of the dichloroethene (tDCE), cis 1,2-dichloroethene (cDCE) and vinyl
long-term test of the PRB, the plume from the emplaced source was
approximately 1 m thick and 1 m wide near the source, with peak
−1
PCE and TCE concentrations of about 50 and 270 mg L , respect-
ively. However, most of the TCM had been dissolved from the
source, resulting in very low concentrations. As shown in Fig. 1, the
PRB was installed 5.5 m downgradient from the emplaced source
and positioned below the water table. The reactive material used
to construct the wall consisted of 22% by weight of granular iron
mix with 78% by weight of coarse sand, and had dimensions of
3
5.5 × 1.6 × 2.2 m, giving a volume of 19.4 m . Permeameter mea-
surements on samples of the iron–sand mixture gave hydraulic
−4
conductivity values of 4.37 × 10 ms −1 (O’Hannesin & Gillham
1998). Metal-enhanced degradation of chlorinated organic com-
pounds is an abiotic redox reaction involving reduction of the
organic compound and oxidation of the metal (Johnson et al. 1996).
The reaction appears to be pseudo-first-order with respect to the
organic concentration and the products of the reaction are chloride,
2+
iron (Fe ) and non-chlorinated, or less chlorinated hydrocarbons
(dichloroethene and vinyl chloride). In the case of chlorinated
ethenes such as PCE and TCE, dechlorination is complete with
ethene and ethane as the final carbon-containing compounds,
while for CTET, a fraction of the parent compound persists as
dichloromethane.
The results of the field experiment are shown in Fig. 2 and
show that for both TCE and PCE there is a substantial decline in
concentration of the core of the contaminant plume at the position
of the first sampling fence (50 cm into the wall), followed by a grad-
ual decrease with further distance into the wall. As a result, TCE
declined from an influent concentration of 268,000 mgL −1 to an
effluent value measured at the 7.5 m fence (50 cm downgradient of
−1
the wall) of 23,350 mgL . Similarly, PCE declined from 58,000 mg
−1
L −1 to 10,970 mgL . Thus, based on the maximum observed con- Fig. 1 (a) Cross-section of the emplaced source of chlorinated
centrations at each sampling fence, 91% of the TCE and 81% of solvents, reactive wall and TCE plume situated in the Borden
the PCE were removed from solution with passage through the aquifer test site, Ontario. (b) Plan view of the permeable wall test
reactive material. It is reasonable to expect that, had a higher per- site, monitoring network and TCE plume. After O’Hannesin and
centage of iron been used in the iron–sand mixture, or had a more Gillham (1998).