Page 274 - Hydrogeology Principles and Practice
P. 274
HYDC07 12/5/05 5:32 PM Page 257
Groundwater pollution remediation and protection 257
BO X
Continued
7. 3
chloride (VC). Of these, the major product within the iron–sand mix-
−1
ture was cDCE (2110 mgL ), with substantially lesser amounts of
−1
−1
1,1-DCE (453 mgL ) and tDCE (146 mgL ); the sum of which is
equivalent to about 1% of the influent TCE. However, measure-
ments of the effluent leaving the wall showed that the DCE isomers
were also degraded within the PRB. Concentrations of VC above the
limit of the analytical method were not detected.
Changes in water chemistry as a result of abiotic reduction of
0
organic compounds involve the oxidation of zero valent iron (Fe )
2+
+
−
by water-producing Fe , an increase in H and OH (eqs 1 and 2),
and a decrease in redox potential and dissolved oxygen. The H +
−
forms hydrogen gas and the OH remaining in solution causes an
increase in pH that can cause precipitation of iron hydroxides and
carbonate minerals (eqs 4–6).
2+
0
2Fe + O + 2H O → 2Fe + 4OH − (aerobic conditions) eq. 1
2
2
2+
0
Fe + 2H O → Fe + H + 2OH − (anaerobic conditions) eq. 2
2
2
2+
−
Fe + 2OH → Fe(OH) 2 (iron hydroxide) eq. 3
−
−
2−
HCO + OH → CO + H O eq. 4
2
3
3
2−
2+
Fe + CO → FeCO 3 (siderite) eq. 5
3
2−
2+
Ca + CO → CaCO 3 (calcite) eq. 6
3
The field results showed that iron concentrations entering the wall
−1
were <0.5 mg L , while within the PRB concentrations were gener-
−1
ally within the range of 5–10 mg L before decreasing to <0.5 mg
L −1 downgradient of the wall. Dissolved oxygen and Eh values
within the treatment zone were nearly always recorded as zero and
within the range −200 to −350 mV, respectively. The pH increased
from a background value of 8.0 to 8.7 in the PRB as a result of the
reduction of water. After 4 years of operation, only trace amounts of
iron oxides and calcium and iron carbonates were found within the
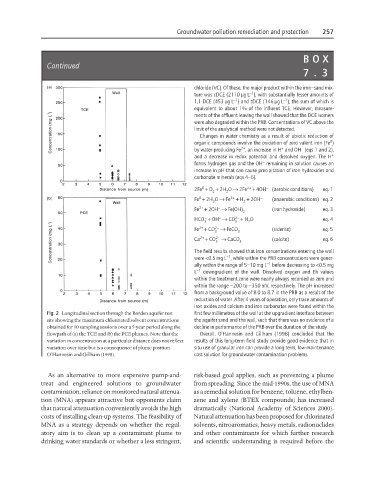
Fig. 2 Longitudinal section through the Borden aquifer test first few millimetres of the wall at the upgradient interface between
site showing the maximum chlorinated solvent concentrations the aquifer sand and the wall, such that there was no evidence of a
obtained for 10 sampling sessions over a 5-year period along the decline in performance of the PRB over the duration of the study.
flowpath of (a) the TCE and (b) the PCE plumes. Note that the Overall, O’Hannesin and Gillham (1998) concluded that the
variation in concentration at a particular distance does not reflect results of this long-term field study provide good evidence that in
variation over time but is a consequence of plume position. situ use of granular iron can provide a long-term, low-maintenance
O’Hannesin and Gillham (1998). cost solution for groundwater contamination problems.
As an alternative to more expensive pump-and- risk-based goal applies, such as preventing a plume
treat and engineered solutions to groundwater from spreading. Since the mid-1990s, the use of MNA
contamination, reliance on monitored natural attenua- as a remedial solution for benzene, toluene, ethylben-
tion (MNA) appears attractive but opponents claim zene and xylene (BTEX compounds) has increased
that natural attenuation conveniently avoids the high dramatically (National Academy of Sciences 2000).
costs of installing clean-up systems. The feasibility of Natural attenuation has been proposed for chlorinated
MNA as a strategy depends on whether the regul- solvents, nitroaromatics, heavy metals, radionuclides
atory aim is to clean up a contaminant plume to and other contaminants for which further research
drinking water standards or whether a less stringent, and scientific understanding is required before the