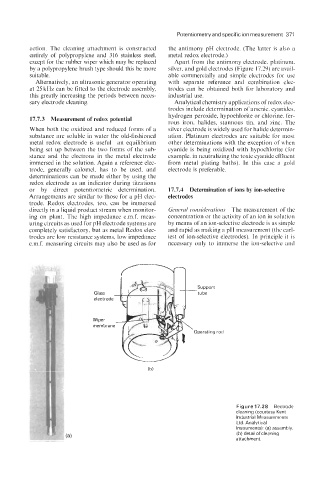
Page 388 - Instrumentation Reference Book 3E
P. 388
Potentiometry and specific ion measurement 371
action. The cleaning attachment is constructed the antimony pH electrode. (The latter is also a
entirely of polypropylene and 316 stainless steel, metal redox electrode.)
except for the rubber wiper which may be replaced Apart from the antimony electrode, platinum,
by a polypropylene brush type should this be more silver, and gold electrodes (Figure 17.29) are avail-
suitable. able commercially and simple electrodes for use
Alternatively, an ultrasonic generator operating with separate reference and combination elec-
at 25 kHz can be fitted to the electrode assembly, trodes can be obtained both for laboratory and
this greatly increasing the periods between neces- industrial use.
sary electrode cleaning. Analytical chemistry applications of redox elec-
trodes include determination of arsenic, cyanides,
hydrogen peroxide, hypochlorite or chlorine. fer-
17.7.3 Measurement of redox potential
rous iron, halides, stannous tin, and zinc. The
When both the oxidized and reduced forms of a silver electrode is widely used for halide determin-
substance are soluble in water the old-fashioned ation. Platinum electrodes are suitable for most
metal redox electrode is useful-an equilibrium other determinations with the exception of when
being set up between the two forms of the sub- cyanide is being oxidized with hypochlorite (for
stance and the electrons in the metal electrode example, in neutralizing the toxic cyanide effluent
immersed in the solution. Again a reference elec- from metal plating baths). In this case a gold
trode, generally calomel, has to be used, and electrode is preferable.
determinations can be made either by using the
redox electrode as an indicator during titrations
or by direct potentiometric determination. 17.7.4 Determination of ions by ion-selective
Arrangements are similar to those for a pH elec- electrodes
trode. Redox electrodes, too, can be immersed
directly in a liquid product stream when monitor- Generul considerations The measurement of the
ing on plant. The high impedance e.m.f. meas- concentration or the activity of an ion in solution
uring circuits as used for pH electrode systems are by means of an ion-selective electrode is as simple
completely satisfactory, but as metal Redox elec- and rapid as making a pH measurement (the earl-
trodes are low resistance systems, low impedance iest of ion-selective electrodes). In principle it is
e.m.f. measuring circuits may also be used as for necessary only to immerse the ion-selective and
Glass -
electrode
Wiper -
membrane
rod
Figure 17.28 Electrode
cleaning (courtesy Kent
Industrial Measurements
Ltd. Analytical
Instruments): (a) assembly,
(b) detail of cleaning
attachment.